A kind of resistant treatment called illusory antigen receptor (Vehicle)-lymphocyte treatment has upset the therapy of numerous sorts of blood malignant growths yet has shown limited viability against glioblastoma — the deadliest sort of essential mind disease — and other strong cancers.
A new exploration driven by examiners at Massachusetts General Hospital (MGH) and distributed in the Diary for ImmunoTherapy of Malignant Growth, Walk 10 in 2023, recommends that sedates that correct irregularities in a strong growth’s veins can work on the conveyance and capability of vehicle white blood cell treatment.
With vehicle lymphocyte treatment, safe cells are taken from a patient’s blood and are changed in the lab by adding a quality for a receptor that trains the cells to connect to a particular protein on the malignant growth cells’ film.
“One of the key reasons that CAR-T therapy hasn’t worked effectively against solid tumors is that intravenously delivered cells are only capable of moving to the invasive margins of a tumor or to limited parts of the tumor,”
Senior author Rakesh K. Jain, Ph.D.,
“One of the principal reasons that Vehicle T treatment hasn’t functioned admirably against strong cancers is that intravenously controlled cells are just fit for moving to either the obtrusive edges of a growth or just in the restricted region of the cancer,” says senior creator Rakesh K. Jain, Ph.D., overseer of the E.L. Steele Research Centers for Growth Science at MGH and the Andrew Werk Cook Teacher of Radiation Oncology at Harvard Clinical School.
“Additionally, growths establish a climate around them that is immunosuppressive, that safeguards them from vehicle T treatment and other enemy of disease medicines directed intravenously through the blood supply.”
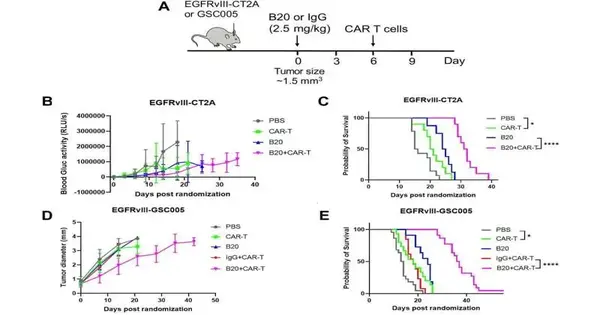
Credit: Diary for Immunotherapy of Malignant Growth (2023) DOI: 10.1136/jitc-2022-005583
Jain and his associates recently showed that “normalizing” cancer’s veins with specialists called enemies of angiogenesis drugs, initially created to hinder the development of fresh blood vessels, can work on the conveyance and against the disease capabilities of invulnerable cells normally delivered by the body.
“We then investigated whether we could further develop Vehicle Lymphocyte penetration and beat obstruction instruments presented by the unusual cancer microenvironment by normalizing glioblastoma veins utilizing a neutralizer that inhibits a significant angiogenic particle called vascular endothelial development factor, or VEGF,” Jain explains.
Utilizing best-in-class live imaging to follow the development of vehicle lymphocytes into cancers continuously, the group found that therapy with an immunizer against VEGF worked on the penetration of vehicle white blood cells into glioblastoma growths in mice. The treatment additionally restrained cancer development and delayed endurance in mice with glioblastoma.
“Considering that an inhibitor of the VEGF immune response, bevacizumab, has been supported for glioblastoma patients and that few vehicle T treatments are being tried in patients, our outcomes give a convincing reason to test the blend of vascular normalizing specialists, like an inhibitor of the VEGF immune response, with current vehicle T treatments,” says Jain. “Furthermore, our methodology may likewise further develop Vehicle T treatment against other strong growths. Thusly, we intend to stretch out our exploration to different cancers.”
Extra review creators incorporate Xinyue Dong, Jun Ren, Zohreh Amoozgar, Somin Lee, Meenal Datta, Sylvie Roberge, Imprint Duquette, and Dai Fukumura.
More information: Xinyue Dong et al, Anti-VEGF therapy improves EGFR-vIII-CAR-T cell delivery and efficacy in syngeneic glioblastoma models in mice, Journal for ImmunoTherapy of Cancer (2023). DOI: 10.1136/jitc-2022-005583