As the principal clinical trial of the medication novobiocin is going to be opened for patients with malignant growths conveying BRCA quality transformations, new exploration at the Dana-Farber Cancer Institute shows the medication represents a twofold danger to cancer cells.
As revealed today in Nature Correspondences, the specialists found that as well as targeting the internal functions of BRCA-transformed cells, novobiocin likewise prompts a safe framework assault on the cells. The discoveries recommend that regardless of whether novobiocin demonstrates as much power as trusted in clinical preliminary studies, it might work fundamentally better whenever matched with drugs that amplify the safe reaction.
The US Food and Drug Administration approved novobiocin for a clinical trial in patients with malignant tumors who have mutations in the BRCA1 or BRCA2 genes in January. The preliminary, to be driven by Dana-Farber agents, is supposed to begin selecting patients by June 1.
“Novobiocin is an inhibitor of POLθ, a protein basic to the endurance of malignant growths with transformations in BRCA1 or BRCA2,” makes sense of the review’s lead creator, Jeffrey Patterson-Fortin, MD, Ph.D., of Dana-Farber. “From past exploration, we knew that crippling POL with novobiocin killed BRCA1- or BRCA2-freak diseases. Here, we needed to look at the commitment of the safe microenvironment — the insusceptible framework cells inside a cancer — to the anticancer impact of novobiocin.”
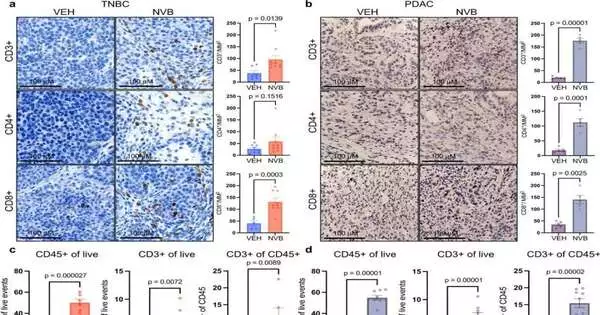
Utilizing two growth models — one of triple-negative bosom disease with BRCA1 changes and one of pancreatic malignant growth with BRCA2 transformations — analysts showed that novobiocin uplifts the pandemonium previously happening in cell division, with deadly ramifications for cancer cells.
They found the medication makes cancer cells become progressively loaded with micronuclei — small scale cores that contain harmed sections of chromosomes. The chromosome pieces slip effectively through holes in the micronuclei layers and into the cells’ cytoplasm, enacting a protein pathway known as cGAS/STING. The pathway causes the body’s battling CD8+ white blood cells to attack the cancers and go into assault mode.
To decide the degree to which this cycle is responsible for the growth cell-killing impact of novobiocin, Patterson-Fortin and his associates drained CD8+ lymphocytes from the two cancer models and found the impact of novobiocin was pointedly decreased. “This shows that the feeling of the resistant framework is a significant piece of novobiocin’s viability, notwithstanding its immediate impact on cancer cells,” says the review’s senior creator, Geoffrey Shapiro, MD, Ph.D., of Dana-Farber.
One of the ways in which the cancer cells adjust to the presence of novobiocin is by increasing their development of the PD-L1 protein, which battles off an immune system microorganism assault, Shapiro proceeds. This recommends that joining novobiocin with a PD-1-hindering specialist could be more viable than novobiocin alone. Specialists desire to send off a clinical preliminary of the mix from now on.
More information: Jeffrey Patterson-Fortin et al, Polymerase θ inhibition activates the cGAS-STING pathway and cooperates with immune checkpoint blockade in models of BRCA-deficient cancer, Nature Communications (2023). DOI: 10.1038/s41467-023-37096-6. www.nature.com/articles/s41467-023-37096-6