All cells emit nanoscale extracellular vesicles, normally as lipid-bilayer-delimited particles. Thus, they are legitimate biomarkers to distinguish various infections.
It is essential to effectively segregate little extracellular vesicles while keeping up with the yield and virtue to investigate their likely analytic, prognostic, and helpful applications.
Traditional techniques for confinement have deficiencies, which incorporate low immaculateness and yield, extensive extraction methods, particular hardware, and significant expenses.
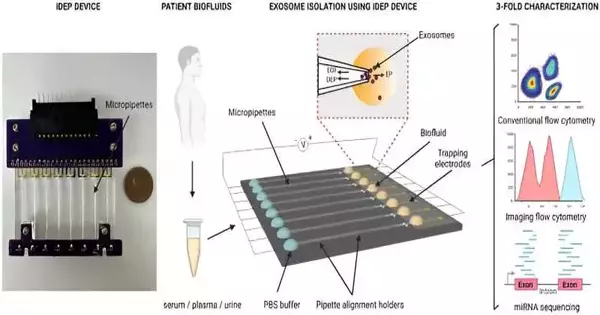
In a review distributed in Logical Reports, Manju Sharma and a group of researchers in biomedical design at the College of Cincinnati, Ohio, U.S., fostered another protector-based dielectrophoretic gadget to quickly separate little extracellular vesicles from biofluids and cell culture media in light of their dielectric properties.
The researchers portrayed the little extracellular vesicles confined to the biofluids of disease patients utilizing the instrument and directed a three-overlay portrayal with ordinary stream cytometry, high-level imaging stream cytometry, and microRNA sequencing to get a high return of unadulterated extracellular vesicles. The stage is productive at quickly disconnecting biomarkers and keeping up with the biomolecular honesty of the vesicles.
Film-typified organic vessels
Naturally, little extracellular vesicles are layer-typified organic vessels found in biofluids, for example, blood, pee, spit, semen, bosom milk, and cerebrospinal liquid, delivered by cells into the extracellular space.
Such nanoscale vesicles can evenly move their biomolecular freight to work as intercellular flagging vectors. Such extracellular vesicles have a serious level of responsiveness and explicitness because of their fantastic soundness. Their initial identification in fluid biopsies can work on the recognition of malignant growths, contaminations, and neurodegenerative and metabolic illnesses.
The disengagement of the vesicles is, be that as it may, testing due to their nanoscale size and physicochemical properties. Detachment techniques commonly rely upon the properties of the extracellular vesicles, and keeping in mind that such gadgets have promising traits, their expense of manufacture, test weakening, and defenselessness to stopping up are inborn difficulties.
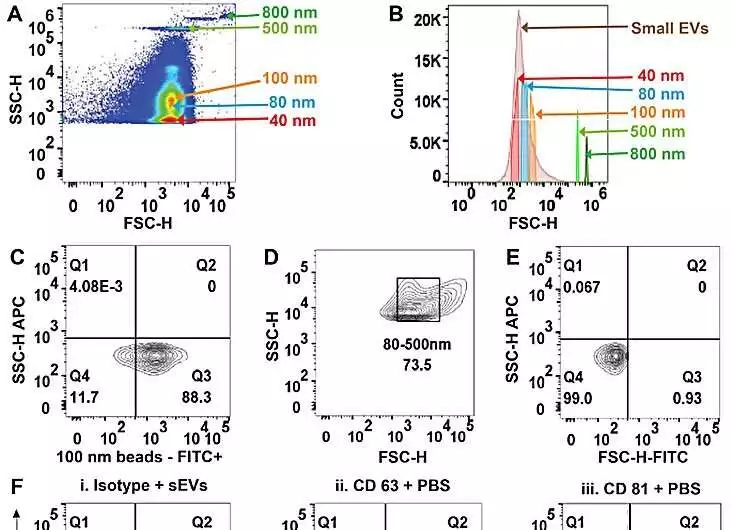
(A) FSC-H versus SSC-H of PS-COOH dots examination on high-goal stream cytometry addresses the goal of dabs size in the PS-COOH blend. (B) Histogram outlining various sizes of PS-COOH dabs and serum-determined little extracellular vesicles (sEVs). (C) Agent plots of PS-COOH-100 nm globule fluorescence (FITC). (D) Agent FSC-H versus SSC-H of sEVs separates from human plasma. (E) Agent FSC-H versus SSC-H of impeccable sEVs showing gating. (F) Agent plots as bad controls I. Isotype-stained sEVs. ii. CD63 immune response in PBS; and iii. CD81 immune response in PBS. (G) Delegate speck plots of sEVs smudged for CD63 from I. serum, II. plasma, and III. pee. (H) Delegate plots of sEVs smudged for CD81 from I. serum, II. plasma, and III. pee. Credit: Logical Reports, doi: 10.1038/s41598-023-45409-4
Accordingly, Sharma and partners fostered a new encasing-based dielectrophoretic approach with micropillars in microfluidic channels to quickly overwhelm nanoparticles in light of their size and remarkable dielectric properties.
Instrument of activity
The gadget kept a variety of micropipettes that are equipped for detaching nanoparticles from small example volumes by applying a fundamentally low electric field across the length of the pipettes. The design of the pore math permitted the separation of extracellular vesicles from small, for example, volumes of adapted cell culture media and biofluids from sound benefactors.
In this work, Sharma and colleagues detached the biofluids of malignant growth patients, which included serum, plasma, and pee, followed by multiparametric portrayal through stream cytometry and cutting-edge miRNA sequencing.
The group sanitized little extracellular vesicles from serum, plasma, and pee in phosphate-cradled saline by utilizing the protector-based dielectrophoretic approach. Sharma and partners utilized transmission electron microscopy to affirm the presence of the vesicles and investigated multiparametric examination of filtered, circling little extracellular vesicles by means of stream cytometry.
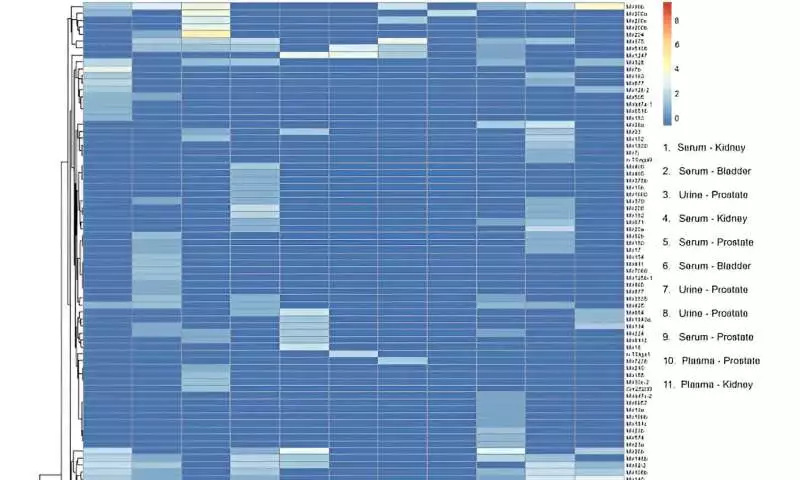
The group segregated the vesicles and dissected them, followed by traditional stream cytometry studies. The specialists further showed the limits and utilization of the gadget by portraying the confines through ImageStream programming.
After miRNA sequencing, the group planned to add 137 unmistakable, mature miRNA records to the human genome across tests to remember the gadget for miRNA biomarker examination work processes. They directed transcriptomic profiles and performed head-part examinations.
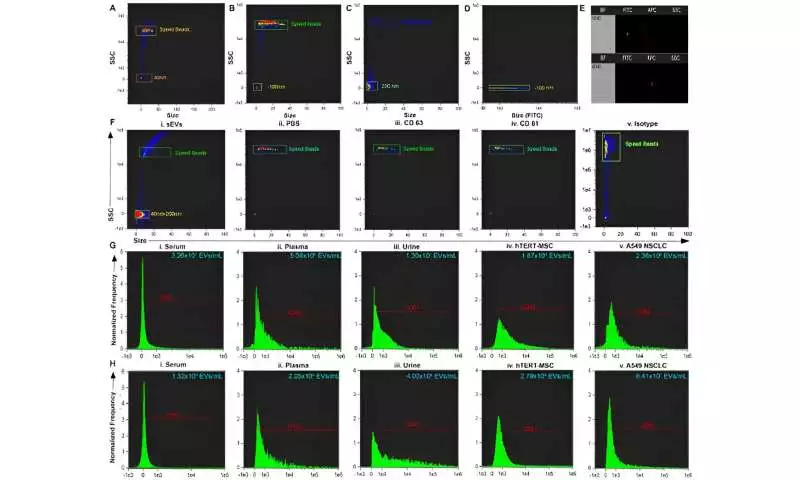
a scaled, focused head-part investigation of exosomal miRNA profiles. Tests bunched in light of the exosomal beginning. Green ran oval features tests separated from plasma. Pink ran circle features tests separated from serum. Dim blue ran oval features tests removed from pee. Credit: Logical Reports, doi: 10.1038/s41598-023-45409-4
Standpoint
Along these lines, Manju Sharma and partners showed the limit and proficiency of a low-voltage, name-free protector-based dielectrophoretic gadget to disconnect little extracellular vesicles from serum, plasma, and pee from malignant growth patients through sub-micron molecule location and multiparametric portrayal by utilizing ordinary stream cytometry and high-level stream cytometry techniques.
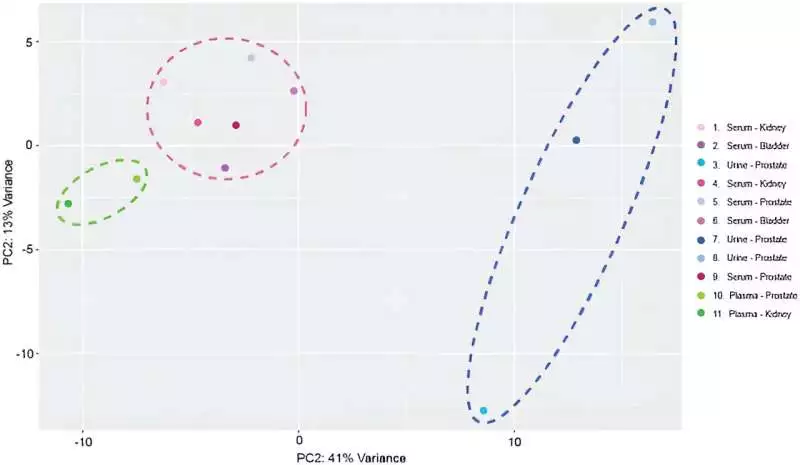
Scaled-centered principal component analysis of exosomal miRNA profiles. Samples were clustered based on exosomal origin. Green dashed oval highlights samples extracted from plasma. Pink dashed circle highlights samples extracted from serum. Dark blue dashed oval highlights samples extracted from urine. Credit: Scientific Reports, doi: 10.1038/s41598-023-45409-4
The RNA centralizations of the work were practically identical to past works and confirmed the detachment strategy to be a feasible option in contrast to those generally settled in the lab. The scientific techniques can be helpful as fluid biopsy stages to confine little extracellular vesicles and foster extracellular vesicle-based demonstrative and checking stages.
More information: Manju Sharma et al, Rapid purification and multiparametric characterization of circulating small extracellular vesicles utilizing a label-free lab-on-a-chip device, Scientific Reports (2023). DOI: 10.1038/s41598-023-45409-4