The treatment of infections is severely hampered by biofilms, which are communities of bacteria that are extremely resistant to antibiotics. While contemplating biofilm arrangement in research facility conditions has been widely directed, understanding their advancement in the perplexing climate of the human respiratory tract has remained slippery.
A group of specialists led by Alexandre Persat at EPFL have now broken the issue by effectively creating organoids called AirGels. Organoids are smaller than usual, self-coordinated 3D tissues developed from undifferentiated cells to mirror real body tissues and organs in the human body. They enable researchers to replicate and investigate the intricate environments of organs in the laboratory, marking a paradigm shift in the field.
Created by Tamara Rossy and her partners, the AirGels are bioengineered models of human lung tissue that open up additional opportunities in contamination research. They upset disease research by precisely copying the physiological properties of the aviation route mucosa, including bodily fluid discharge and ciliary beating. This innovation permits researchers to concentrate on aviation route diseases in a more practical and extensive way, overcoming any barrier between in vitro examinations and clinical perceptions.
“There is much to say about this study, but engineering organoids for infection research has enormous potential. It completely changes the game.”
Alexandre Persat at EPFL.
According to Persat, “there is a lot to say about this study, but the engineering of organoids for research on infections has tremendous potential.” It changes everything.”
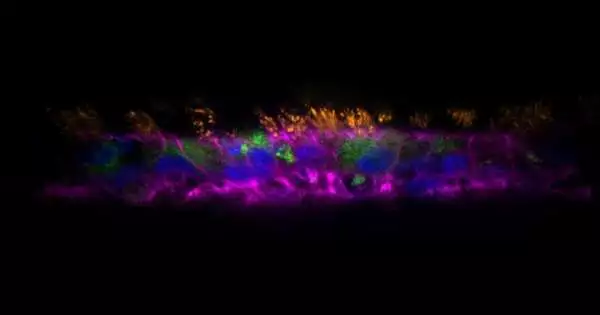
In the review, distributed in PLoS Science, the scientists utilized AirGels to explore the job of bodily fluid during the time spent on biofilm development by Pseudomonas aeruginosa, a pathogenic bacterium that is usually impervious to anti-toxins. By contaminating the AirGels with P. aeruginosa and concentrating on them under high-goal live microscopy, they had the option to structure biofilms progressively.
Their perceptions uncovered that P. aeruginosa effectively incites the withdrawal of its host’s bodily fluid utilizing retractile fibers known as type IV pili (T4P). The T4P fibers create the vital power to get the aviation route’s bodily fluid, which permits P. aeruginosa cells to total and shape a biofilm. The specialists approved their discoveries with follow-up reproductions and biophysical investigations of chosen P. aeruginosa freaks.
The study uncovered a previously unknown mechanism that contributes to the formation of biofilm in the respiratory tract, demonstrating that the AirGel organoid model can provide unique insights into the mechanical interactions between bacteria and their hosts’ environments.
Having the option to design organoids that steadfastly repeat the mucosal climate opens up new roads of investigation, empowering analysts to reveal disregarded parts of diseases, researching the impact of extra physiological elements like temperature, stickiness, medications, and substance stressors on the turn of events and movement of contamination, and fostering designated medicines against anti-infection safe microorganisms.
More information: PLoS Biology (2023). DOI: 10.1371/journal.pbio.3002209