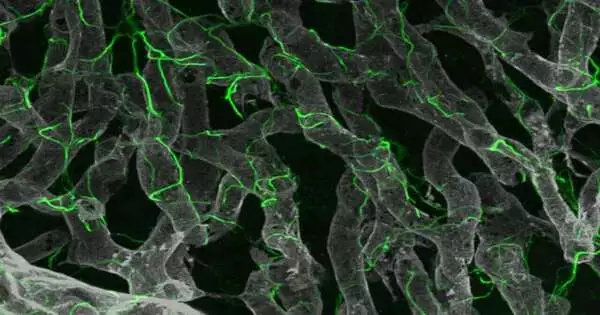
Analysts discovered novel applicant drug targets for non-alcoholic fatty liver disease (NAFLD) by utilizing the most recent innovations, including both single-atomic sequencing of mouse and human liver tissue and high-level 3D glass imaging of mice to portray key scar-creating liver cells.The examination was driven by agents at the Icahn Institute of Medicine at Mount Sinai.
Using these inventive methods, the researchers discovered a network of cell-to-cell communication that promotes scarring as liver disease progresses.The discoveries, published web-based on January 4 in Science Translational Medicine, could prompt new medicines.
Described by fat in the liver and frequently connected with type 2 diabetes, hypertension, and raised blood lipids, NAFLD is an overall danger. In the United States, 30 to 40% of adults are estimated to be affected, with approximately 20% of these patients having a more advanced stage called non-alcoholic steatohepatitis, or NASH, which is distinguished by liver irritation and can progress to cutting edge scarring (cirrhosis) and liver disappointment.
“By researching hepatic stellate cells, which are the main scar-producing cells in the liver, we hoped to understand the basis of this fibrotic scarring and find pharmacological targets that could lead to new treatments for advanced NASH.”
Senior study author Scott L. Friedman, MD, Irene and Dr. Arthur M. Fishberg Professor of Medicine,
NASH is likewise the fastest-rising reason for liver disease around the world. Because the advanced stages of NASH are caused by the accumulation of fibrosis or scarring, efforts to prevent fibrosis are at the center of efforts to treat NASH, yet no medications are currently endorsed for this reason, say the agents.
As a feature of the tests, the scientists performed single-atomic sequencing in equal investigations of both mouse models of NASH and human liver tissue from nine subjects with NASH and two controls. They discovered a total of 68 sets of potential medication focuses shared by the two species.Besides, the examiners sought after one of these sets by testing a current disease drug in mice as a proof of concept.
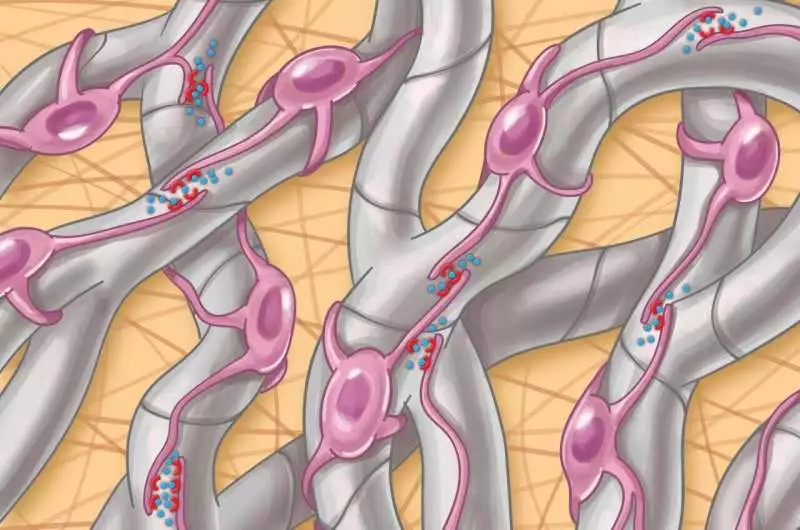
Hepatic stellate cell autocrine flagging (connections among themselves) in NASH is worked with by actual cell contacts through their projections.
“We meant to comprehend the premise of this fibrotic scarring and recognize drug targets that could prompt new medicines for cutting-edge NASH by concentrating on hepatic stellate cells, which are the key scar-creating cells in the liver,” said senior review creator Scott L. Friedman, MD, Irene and Dr. Arthur M. Fishberg, Teacher of Medication, Dignitary for Helpful Revelation, and Head of Liver Illnesses at Icahn Mount Sinai. “By combining this new glass liver imaging approach—a high level tissue clearing strategy that enables profound knowledge—with quality articulation examination in individual stellate cells, we have revealed an entirely new comprehension of how these cells create scarring as NASH progresses to late stages.”
The scientists found that in cutting-edge illnesses, stellate cells foster a thick organization, or meshwork, of connections among themselves that work with these 68 novel cooperation matches not previously recognized in this sickness.
“We affirmed the significance of one such set of proteins, NTF3-NTRK3, utilizing a particle previously created to hinder NTRK3 in human tumors and reusing it to lay out its true capacity as another medication to battle NASH fibrosis,” said first writer Shuang (Sammi) Wang, Ph.D., a teacher in the Division of Liver Illnesses. “This new comprehension of fibrosis improvement proposes that exceptional fibrosis might have a novel collection of signs that speed up scarring, which address a formerly unnoticed arrangement of medication targets.”
The researchers believe that the hardware of how cells communicate with one another evolves as the disease progresses, so some medications may be more effective early on and others later on. Also, a similar medication may not work for all phases of illness.
As of now, the agents are working with Icahn Mount Sinai physicists to additionally enhance NTRK3 inhibitors for the treatment of liver fibrosis. Then, the examiners plan to practically screen all competitor interactors in a phone culture framework, followed by testing in preclinical models of liver illness, as they have accomplished for NTRK3. Also, they desire to stretch out their endeavors to decide whether comparable connections among fibrogenic cells underlie the fibrosis of different tissues, including the heart, lung, and kidneys.
“An autocrine flagging circuit in hepatic stellate cells underpins progressed fibrosis in non-alcoholic steatohepatitis,” according to the paper’s title.
More information: Shuang Wang et al, An autocrine signaling circuit in hepatic stellate cells underlies advanced fibrosis in nonalcoholic steatohepatitis, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.add3949. www.science.org/doi/10.1126/scitranslmed.add3949
Journal information: Science Translational Medicine