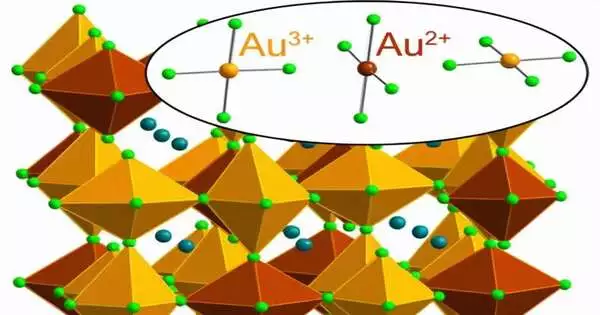
Interestingly, Stanford scientists have figured out how to make and balance out an incredibly uncommon type of gold that has lost two adversely charged electrons, meaning Au2+. The material balancing out this subtle rendition of the esteemed component is a halide perovskite—a class of glasslike materials that holds extraordinary commitment for different applications, including more-productive solar-powered cells, light sources, and gadget parts.
Shockingly, the Au2+ perovskite is additionally fast and easy to make utilizing off-the-rack fixings at room temperature.
“It was a genuine shock that we had the option to combine a steady material containing Au2+—I didn’t completely accept that from the beginning,” said Hemamala Karunadasa, academic administrator of science at the Stanford School of Humanities and Sciences and senior creator of the review distributed Aug. 28 in Nature Science. “Making this first-of-its-sort Au2+ perovskite is energizing. The gold particles in the perovskite bear solid similarities to the copper molecules in high-temperature superconductors, and weighty iotas with unpaired electrons, as Au2+, show cool, attractive impacts not found in lighter molecules.”
“Halide perovskites have truly alluring properties for the majority of ordinary applications, so we’ve been hoping to extend this group of materials,” said Kurt Lindquist, the lead creator of the review, who led the examination as a Stanford doctoral understudy and is currently a postdoctoral researcher in inorganic science at Princeton College. “An exceptional Au2+ perovskite could open a few captivating new roads.”
“It was a complete surprise that we were able to synthesize a stable material containing Au2+—I couldn’t believe it at first. Creating this first-of-its-kind Au2+ perovskite is amazing. The gold atoms in perovskite are quite similar to copper atoms in high-temperature superconductors, and heavier atoms with unpaired electrons, such as Au2+, exhibit cool magnetic phenomena that lighter atoms do not.”
said Hemamala Karunadasa, associate professor of chemistry at the Stanford School of Humanities and Sciences and senior author of the study published Aug. 28 in Nature Chemistry.
Weighty electrons in gold
As a basic metal, gold has for some time been esteemed for its general shortage as well as its unequaled pliability and substance idleness, meaning it tends to be effortlessly molded into adornments and coins that don’t respond to synthetic compounds in the climate and stain over the long run. An extra key justification for its worth is gold’s namesake tone; seemingly no other metal in its unadulterated state has such a particularly rich tint.
The crucial physical science behind gold’s acclaimed appearance likewise makes sense of why Au2+ is so uncommon, as Karunadasa made sense of.
The root reason is relativistic impacts, initially hypothesized in Albert Einstein’s renowned hypothesis of relativity. “Einstein instructed us that when items move exceptionally quickly and their speed moves toward a critical part of the speed of light, the items get heavier,” Karunadasa said.
This peculiarity applies to particles as well and has significant ramifications for “gigantic” weighty components, for example, gold, whose nuclear cores brag countless protons. These particles on the whole apply tremendous positive charge, constraining adversely charged electrons to spin around the core dangerously fast. As a result, the electrons become heavier and firmly encompass the core, dulling its charge and permitting external electrons to float farther than in ordinary metals. This revision of electrons and their energy levels prompts gold-engrossing blue light and thusly seems yellow to our eye.
On account of the plan of gold’s electrons, because of relativity, the molecule normally happens as Au1+ and Au3+, losing one or three electrons individually and rejecting Au2+. (The “2+” shows a net positive charge from the deficiency of two adversely charged electrons, and the “Au” compound image for gold hails from “aurum,” the Latin word for gold.)
A press of L-ascorbic acid
With the perfect atomic arrangement, Au2+ can persevere, the Stanford analysts found. Lindquist said he “coincidentally found” the new Au2+-holding onto perovskite while working on a more extensive undertaking fixated on attractive semiconductors for use in electronic gadgets.
Lindquist blended a salt-assembled cesium chloride and Au3+-chloride in water and added hydrochloric corrosive to the arrangement “with a little L-ascorbic acid tossed in,” he said. In the following response, L-ascorbic acid (a corrosive) gives an (adversely charged) electron to the normal Au3+, shaping Au2+. Intriguingly, Au2+ is steady in the strong perovskite yet not in arrangement.
“In the lab, we can make this material involving extremely basic fixings in around five minutes at room temperature,” said Lindquist. “We end up with a powder that is extremely dull green, almost dark, and shockingly weighty in light of the gold it contains.”
Perceiving that they might have hit new science with the jackpot, in a manner of speaking, Lindquist played out various tests on the perovskite, including spectroscopy and X-beam diffraction, to research how it retains light and to describe its gem structure. Stanford research bunches in physical science and science, led by Youthful Lee, teacher of applied physical science and of photon science, and Edward Solomon, the Monroe E. Spaght teacher of science and teacher of photon science, further added to concentrating on the way of behaving of Au2+.
The examinations eventually confirmed the presence of Au2+ in a perovskite and, all the while, added a part to the extremely old story of science and physical science, including Linus Pauling, who got the Nobel Prize in Science in 1954 and the Nobel Harmony Prize in 1962. Right off the bat, in his profession, he dealt with gold perovskites containing the normal structures Au1+ and Au3+. Incidentally, Pauling likewise later concentrated on the design of L-ascorbic acid—one of the fixings expected to yield a stable perovskite containing the slippery Au2+.
“We love Linus Pauling’s association with our work,” Karunadasa said. “The union of these perovskites makes for a decent story.”
Looking forward, Karunadasa, Lindquist, and associates intend to study the new material further and change its science. The expectation is that an Au2+ perovskite can be utilized in applications that require attraction and conductivity as electrons jump from Au2+ to Au3+ in the perovskite.
“We’re eager to investigate what an Au2+ perovskite could do,” Karunadasa said.
More information: Kurt P. Lindquist et al, Stabilizing Au2+ in a mixed-valence 3D halide perovskite, Nature Chemistry (2023). DOI: 10.1038/s41557-023-01305-y