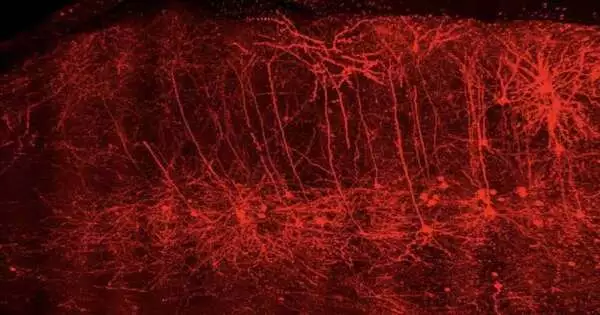
A group of neuroscientists at the Clinical College of South Carolina (MUSC) has recognized changes in the movement of synapses known as pyramidal neurons, which add to what medicate is looking for in a preclinical model of narcotic use issues. These neurons became more excited when heroin access was restricted. The movement of these neurons was reestablished to typical levels by impeding the chemical protein kinase A (PKA). Restraining this catalyst additionally decreased narcotic looking for conduct. The findings of their team were recently published in the Journal of Neuroscience by Jacqueline McGinty, Ph.D., a professor of neuroscience, and Saurabh Kokane, Ph.D., a postdoctoral scholar in McGinty’s laboratory.
The gamble of narcotic excess can increment upon return to medicate looking for and using it, or backslide after a time of not utilizing the medication or restraint.
Kokane stated, “The key to the successful development of effective treatments for substance use disorders is the prevention of return to use.”
“When drug cues in the environment become overpowering, alterations in the recovered addict’s brain cause them to relapse, albeit the precise nature of these changes is still unknown.”
Jacqueline McGinty, Ph.D., professor of neuroscience,
“Following quite a while of examination on the narcotic use issue, three FDA-supported drugs exist, yet they just diminish the seriousness of detoxification side effects and don’t stop the return to use.” As a result, more treatment options are absolutely necessary,” he stated. Presently, we miss the mark on thorough comprehension of the impacts of narcotics, similar to heroin, on the neurons that drive return to use. There may be more options for treatment if these changes are better understood.”
At MUSC, the McGinty Lab discovered specific types of pyramidal neurons that are associated with relapse. During abstinence from heroin, a common opioid, they discovered that these neurons in the prelimbic prefrontal cortex of the brain undergo molecular and functional changes that disrupt their function. The prelimbic cortex is one of the regions of the cerebrum associated with navigation and motor control. This part of the brain can be disrupted by opioids, which can cause compulsive drug seeking in people with opioid use disorders.
Importantly, the researchers in this preclinical study demonstrate in a rodent model that drug seeking can be avoided by restoring normal function to these neurons by inhibiting a key enzyme during heroin abstinence.
Figuring out backslides
Substance use issues are constant, treatable circumstances from which individuals can recover. Part of these disorders is continuing to use drugs despite the negative effects, as well as periods of abstinence that are followed by a return to use, or relapse.
One sort of sign-prompted backslide can happen when somebody with a substance use disorder experiences a “signal” or “trigger” that might lead that person to hunger for the utilization of a medication. A person with an alcohol use disorder, for instance, may experience a craving for a drink when they hear the sound of champagne corks popping, and someone with an opioid use disorder may experience a craving for heroin when they see people using drugs on television.
Kokane stated, “These overwhelming cravings may lead those with opioid use disorders to use again, even if they do not want to.”
“The difficulties with enthusiastic medication use are the deficiency of the capacity to settle on various conduct choices and the absence of protection from natural upgrades that help you remember taking a narcotic like heroin,” McGinty said.
Letting completely go
Changes in a few mind districts are liable for prompt actuated backslides and make it hard for an individual with substance use turmoil to control drug desires. Kokane and the McGinty Lab focused on two of these regions in this study: the core accumbens and the prelimbic cortex.
“The core accumbens is a mind region that gets input from the prelimbic cortex and from dopamine-delivering pathways that make the longing to take the substance again that is related to all habit-forming drugs, including narcotics,” made sense of Kokane. Abnormalities in these pathways’ operation during abstinence are a major cause of cue-induced relapse.
In most cases, the decision to act on a feeling or desire is made by the prelimbic cortex and other cortical regions. According to Kokane, the prelimbic cortex either motivates us to stop acting or pushes us to act through its connections with the nucleus accumbens.
Opioids cause distinct changes in these brain regions’ function that make quitting opioid use much more difficult.
According to McGinty, “the exact types of changes that occur have not been fully studied.” “The changes in the brain of someone recovering from substance use disorder drive a return to use when environmental drug cues become overwhelming.”
The MUSC team discovered, employing a rodent model, that heroin abstinence increases the activity of neurons connecting the prelimbic cortex to the nucleus accumbens. The increased activity of these neurons may assist the nucleus accumbens in driving relapse rather than halting it.
The use of drugs may then continue unchecked, frequently despite the negative social and psychological effects.
Restoring control Although additional research is required, Kokane and McGinty believe that restoring normal activity in the neurons of the prelimbic cortex may prevent cue-induced relapse.
Kokane stated, “We need to understand in greater detail the changes that occur in neurons during abstinence from heroin and determine how they lead to relapse.”
The MUSC investigation additionally discovered that a compound, PKA, is more dynamic during heroin forbearance. In the prelimbic cortex, where restraint expanded neuronal movement, scientists found that obstructing PKA privately returned neuronal action to typical levels.
The MUSC researchers came up with a novel idea as a result of this finding: maybe PKA restraint could reestablish control.
Kokane stated, “During heroin abstinence, we saw a decrease in cue-induced relapse when we infused the PKA inhibitor into the prelimbic cortex.”
By hindering PKA, the specialists have found one method for reestablishing control over the prelimbic cortex during restraint from narcotics in a rat model. Importantly, because heroin seeking was reduced, regaining brain control also resulted in improved behavioral control.
Kokane stated, “Our findings provide a novel molecular target for developing future pharmacotherapies.” We are at a beginning phase in this examination, but it has potential. Our findings suggest that pharmacotherapies that specifically target functional changes that occur in particular types of neurons during heroin abstinence, such as those we identified in the prelimbic cortex, should be the focus of future research.”
Up to that point, the group is eager to proceed with its preclinical exploration to reveal insight into prelimbic command over narcotic chasing and backslide and to uncover extra targets.
According to McGinty, “It is important to realize that the brain is constantly adapting to the environment” and that “the changes we have documented in the prefrontal cortex during heroin abstinence, although persistent, are not necessarily permanent and are subject to reversal.”
More information: Saurabh S. Kokane et al, Increased Excitability and Synaptic Plasticity of Drd1- and Drd2- expressing Prelimbic Neurons Projecting to Nucleus Accumbens after Heroin Abstinence are Reversed by Cue-induced Relapse and Protein Kinase A Inhibition, The Journal of Neuroscience (2023). DOI: 10.1523/JNEUROSCI.0108-23.2023