Following 30 years of beating results down in endeavoring to foster medications to restrain a changed protein related to a portion of the additional moving diseases to treat, research on RAS proteins is blasting.
New disclosures have reexamined the thought that RAS is an “undruggable” target or that singular RAS changes are vague in their belongings, said MUSC Hollings Disease Center specialist John O’Bryan, Ph.D.
O’Bryan, alongside his long-lasting exploration accomplice Shohei Koide, Ph.D., overseer of Malignant growth Biologics at Perlmutter Disease Center at NYU Langone, and extra colleagues at Hollings and Perlmutter, have now added to this developing group of information with their improvement of engineered monobodies that not just join to KRAS (G12D), a particular RAS transformation that is normal in pancreatic, lung, and colorectal tumors, yet additionally repress a portion of KRAS (G12D’s) activities.
“It could just bind and have no effect. However, it turns out that practically all of the RAS monobodies we’ve created are inhibitory, meaning they bind to critical regions required for RAS function. So, by establishing that they preferentially bind and inhibit RAS, and then determining where they bind, we can acquire insight into how those RAS areas are critical for the protein’s function.”
MUSC Hollings Cancer Center researcher John O’Bryan, Ph.D.
Importantly, the strategy they used to create the monobodies can be used to target other mutations that are currently considered “undruggable.”
Focusing on KRAS
RAS proteins are biochemical on/off switches that control motion for the majority of development components and chemical receptors.
“It’s a truly basic sign transfer in the cell,” O’Bryan said. However, mutations cause RAS to become stuck in the “on” position, which results in uncontrolled growth and, ultimately, cancer.
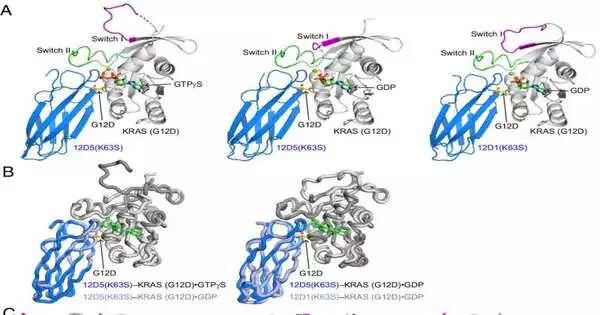
O’Bryan and Koide have been cooperating on RAS for over 10 years. They describe these synthetic monobodies and the structure of a KRAS (G12D) hidden pocket that scientists want to use as a cover for cancer-fighting drugs in a paper that was published in the Proceedings of the National Academy of Sciences.
O’Bryan elaborated, “When you make a monobody, you don’t know what it’s going to do.” It might just bind without doing anything. However, it turns out that almost all of the monobodies we’ve created for RAS are inhibitory—that is, they bind to important RAS-functioning regions. We can therefore gain insight into the significance of those regions of RAS for the protein’s function by first demonstrating that they selectively bind and inhibit RAS and then determining where they bind.”
RAS transformations are available in around 20% of every human malignant growth, O’Bryan said, yet they can be seen in 90% of pancreatic ductal adenocarcinomas (PDAC), the most well-known kind of pancreatic disease.
“That tumor is actually driven by RAS.” It’s one of the first things that started the formation of PDAC,” O’Bryan said.
Their distribution comes while the comprehension of RAS is jumping forward. The Food and Drug Administration has approved two drugs for lung cancer that target KRAS (G12C), a common RAS mutant protein. What’s more, this year, the beginning phase of clinical trials for a medication focusing on KRAS (G12D) started. That medication’s advancement was just conceivable due to the work of Kevan Shokat, Ph.D., who uncovered the secret pocket that these medications target, O’Bryan said.
O’Bryan and Koide, on the other hand, began their work by sorting through libraries of monobodies long before anyone was aware of that secret pocket.
“It turns out our monobody ties around that pocket and really opens it up more,” O’Bryan said. “That suggests that drug design and development may benefit from utilizing that information in some way.”
In their paper, the scientists likewise portray how that secret pocket is organized, which they accept gives significant information to those creating cutting-edge KRAS (G12D) inhibitors.
They likewise accept that the protein-design innovations used to foster their monobodies could be utilized against other testing targets and eventually demonstrate a more clear methodology.
O’Bryan and Koide continued to work on RAS independently and collectively, even as they polished their PNAS paper.
O’Bryan was as of late granted an award by the Branch of Safeguard to deal with conveying monobodies into the lungs as possible treatments, and he is working with Aaron Hobbs, Ph.D., an individual Hollings researcher, whose exploration centers around KRAS (G12R) in pancreatic disease.
More information: Padma Akkapeddi et al, Exploring switch II pocket conformation of KRAS(G12D) with mutant-selective monobody inhibitors, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2302485120