Late advances in imaging innovation have made it conceivable to picture intracellular elements, which offers a superior understanding of a few vital organic standards for speeding up helpful turns of events. Fluorescent naming is one such method that is utilized to recognize intracellular proteins, their elements, and brokenness. Both inner as well as outer tests with fluorescent colors are utilized for this reason, albeit outside tests can more readily visualize intracellular proteins when contrasted with the inward tests. However, their application is limited by a vague restriction to intracellular parts, resulting in low objective explicit flagging and higher foundation clamor.
As of late, a fluorescent-named immunosensor known as Quenchbody (Q-body) has been effectively used to identify antigens in arrangements or on the cell surface. A Q-body is basically an immunizer part with the capacity to bind a particular antigen.
In this scenery, analysts from Japan and Singapore, driven by Prof. Hiroshi Ueda from Tokyo Foundation of Innovation (Tokyo Tech), Japan, as of late revealed the relevance of Q-bodies for imaging of intracellular proteins in live cells. Their discoveries are presently published in Compound Science.
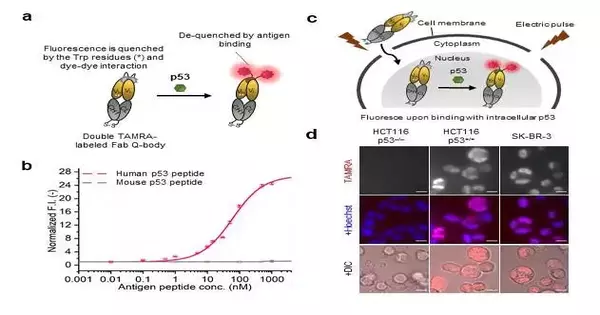
“We expected that because the Q-body is a site-specific and antigen-dependent imaging tool, it will exhibit antigen-dependent switchable fluorescence when interacting with the target protein, allowing precise viewing of intracellular dynamics. We demonstrated this by creating a Q-body for p53, a tumor suppressor biomarker protein involved in DNA repair, cell proliferation, and cell death.”
Prof. Hiroshi Ueda from Tokyo Institute of Technology
“Since the Q-body fills in as a site-explicit and antigen-subordinate imaging device, we guessed that it will show antigen-subordinate switchable fluorescence on connecting with the objective protein, empowering exact perception of intracellular elements. “We showed this by blending a Q-body for p53, a cancer silencer biomarker protein that assumes a significant part in DNA fix, cell division, and cell demise,” makes sense to Prof. Ueda.
The group blended a “twofold” fluorescence color named Q-body called “C11_Fab Q-body,” which showed better responsiveness and target explicitness when contrasted with regular tests in human disease cells communicating p53. Since the outflow of p53 expands in disease cells, they electroporated the Q-body in a few human malignant growth cell lines to confirm their speculation.
Contrasted with a customary test that showed nonstop fluorescence flags even without p53, the Q-body test showed fluorescence signals in “fixed” cells (cells with denatured proteins to end decay) communicating p53. Also, the Q-body test could imagine both wild (control) and freak (p53) types in fixed cell tests.
Further, the group noticed fluorescence signals with an 8-crease higher power in live human colon disease cell lines with p53 articulation when contrasted with the negatives. Strangely, the Q-body was steady in the long haul, showing fluorescence force changes with tentatively prompted changes in p53 levels.
Stream cytometry revealed higher immunofluorescence with Q-body in cells communicating p53. Besides, on arranging, the proportion and fluorescence signal of these cells were altogether higher when contrasted with the others (regardless of Q body).
Prof. According to UEDA, “The current methods can’t give exact imaging of less plentiful intracellular focuses with high explicitness and awareness. In this unique situation, our review shows the capability of Q-bodies in live cell imaging for better perception of dynamical intracellular changes and gives a way to deal with intracellular antigen-explicit arranging of live cells utilizing a Q-body.
Looking forward, we can expect the advancement of a lot more Q-bodies for picturing a few other intracellular biomarkers, providing ways to further develop cell-based helpful turns of events and disease research.
More information: Yancen Dai et al, Intra Q-body: an antibody-based fluorogenic probe for intracellular proteins that allows live cell imaging and sorting, Chemical Science (2022). DOI: 10.1039/d2sc02355e
Journal information: Chemical Science