New Jersey Foundation of Innovation (NJIT) scientists have revealed another lab method they say addresses a “outlook change” in how drug labs test and produce new protein-based drugs. For example, helpful monoclonal antibodies are being created to treat various illnesses, from tumors to irresistible sicknesses.
Scientists say their electrochemistry-based approach, depicted in the journal Logical Science, could consider security and quality testing of promising biotherapeutics to be finished for a portion of the time expected by regular strategies, which commonly require the extended and expensive creation of certain biomaterials utilized for test testing.
The review was led as a team with scientists from Merck, Johnson & Johnson, and Ohio College.
“This strategy we’ve created at NJIT can possibly have a significant effect in quantitative proteomics, and it addresses a change in outlook in the drug industry as far as checking biopharmaceutical items and cycle pollution for quality control,” said Hao Chen, the paper’s lead creator and teacher at NJIT’s Branch of Science and Natural Sciences.
“This approach developed at NJIT has the potential to have a significant influence in quantitative proteomics, and it marks a paradigm shift in the pharmaceutical sector in terms of monitoring biopharmaceutical product and process impurities for quality control,”
Hao Chen, the paper’s corresponding author and professor at NJIT’s Department of Chemistry and Environmental Sciences.
“With this review, we’ve presently shown a methodology that can measure drug items and cycle pollution considerably more rapidly and precisely than had been potential.” We anticipate that it should turn out to be helpful to work with remedial protein and immunization advancement for treatment and avoidance of various illnesses later on.
Generally, such testing, or protein quantitation, includes the tedious planning of engineered isotope-named peptides which are utilized as inner norms to gauge all protein focuses, for example, assisting researchers with effectively checking the viability and security of helpful protein parts all through the medication improvement process.
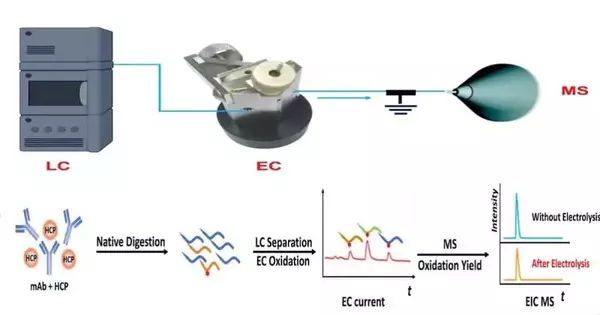
To beat this limit, Chen’s lab developed a coulometric mass spectrometry (CMS) approach for outright quantitation of proteins without the utilization of norms. The strategy instead applies fluid chromatography-mass spectrometry and an electrochemical stream cell to quickly measure and identify changes in target proteins or peptides in view of electrochemical marks.
Rather than trusting that weeks will get norms or reagents in customary methodologies, one could do CMS quantitation tests immediately. Hence, it would work with the following medication pollutions found during the cycle and guarantee their viable leeway with process enhancement and control, “said Chen.
“Such a device permits us to isolate peptides after protein processing with fluid chromatography, screen peptide oxidation in the electrochemical stream cell to create an electric flow, and measure the oxidation yield with mass spectrometry,” made sense of the paper’s most memorable creator and NJIT Ph.D. understudy, Yongling man-made intelligence. “The mix of electric flow signals alongside the oxidation yield gives adequate data for outright quantitation of peptides and proteins.”
In their review, the group showed their CMS strategy by accomplishing outright quantitation of various proteins (-lactoglobulin B, -lactalbumin, and carbonic anhydrase) in a combination in one run without utilizing any norms.
Specifically, the group demonstrated the strategy’s ability to recognize protein deamidation — a common debasement event in useful proteins caused by physical or chemical burdens all through the assembling system and capacity.
The group effectively measured a few protein debasement items, including a vital middle of protein corruption — the development of succinimide — which has never been finished before with outright evaluation because of the absence of norms, as per the review’s creators.
“The absence of norms is brought about by the difficulties in their new blend,” said Chen. “Having the option to precisely measure the deamidation items and intermediates could give us a better understanding of helpful protein corruption and possibly lead to a better approach to exploring illness pathologies and maturing processes.”
Presently, Chen’s lab intends to apply their new strategy for large-scale quantitation of thousands of proteins in a single run. They also intend to improve the awareness of their CMS examination to allow measuring low levels of proteins in complex natural examples, which could aid research endeavors transition from clinical diagnostics and medication disclosure to accuracy medication, for which ID and quantitation of tests at the subatomic level are critical.
“As proteins play out a huge range of capabilities inside creatures, the significance of outright protein quantitation is difficult to exaggerate,” said Chen. “CMS ought to accelerate processes for illness finding, drug revelation, and improvement, and it presently opens another entryway for scholars and natural chemists to investigate amounts of proteins in the human body that might serve significant natural capabilities or jobs as sickness biomarkers and medication targets.”
More information: Yongling Ai et al, Standard-Free Absolute Quantitation of Antibody Deamidation Degradation and Host Cell Proteins by Coulometric Mass Spectrometry, Analytical Chemistry (2022). DOI: 10.1021/acs.analchem.2c02709
Journal information: Analytical Chemistry