In gram-negative microbes, which incorporate the absolute most wrecking human microorganisms, only two systems for the production of polysaccharides have been recognized up to this point. Presently, a Max Planck research group driven by Lotte Sgaard-Andersen has recognized a totally clever third system for how polysaccharides are traded. These discoveries, distributed in mBio, prepare us toward a total understanding of the systems that intervene in the security, motility, and connection of numerous bacterial microbes.
Microbes do not just blossom with sugar as a carbon and energy source—they likewise produce and emit a wide assortment of supposed polysaccharides. Polysaccharides are a series of sugars and are the most plentiful biopolymers on the planet. The long sugar chains play crucial parts in free-living, commensal, and pathogenic microbes. They are likewise vital for bacterial security, sheathing the cells against natural burdens like parching, safe effectors, and hunters. Their glue and primary capabilities add to surface colonization and biofilm arrangement. They are likewise significant for the fruitful use of antibacterial antibodies. Hence, they hold the keys to understanding and controlling both useful and pathogenic human, creature, and plant-organism connections. Also, to wrap things up, polysaccharides are utilized in food, drugs, and clinical ventures.
The polysaccharide trade is a significant test on the grounds that the particles are synthetically different and huge. Until now, only two systems for polysaccharide production in Gram-negative microbes have been identified: an external film OPX protein (in the alleged Wzx/Wzy- and ABC carrier subordinate pathways) and an external layer -barrel protein (in the alleged synthase-subordinate pathways).However, there are instances of pathways that don’t appear to follow these basic plans: External film -barrel proteins, for example, were known to be important for polysaccharide trade in some Wzx/Wzy-pathways, such as in Vibrio cholerae and Myxococcus xanthus, but the specific system was unknown.Also, different examinations depict short OPX proteins that miss the mark on the part that coordinates into the external film. Here, it is unclear the way that these proteins could uphold polysaccharide trade.
“We began by closely examining the M. xanthus Wzx/Wzy-dependent pathway for the synthesis of a secreted polysaccharide known as EPS.”
Dr. María Pérez-Burgos
An exploration group at the Max-Planck-Institute for Terrestrial Microbiology led by Lotte Sgaard-Andersen had the option to reveal new insights into these inquiries. Utilizing tests and computational primary science, the researchers give proof of a completely clever system for how microbes can trade polysaccharides across the external film. Johannes Schwabe, an alumni understudy and lead creator of the review, and Dr. Mara Pérez-Burgos say, “We began by investigating the Wzx/Wzy-subordinate pathway for the union of an emitted polysaccharide called EPS in M. xanthus.”
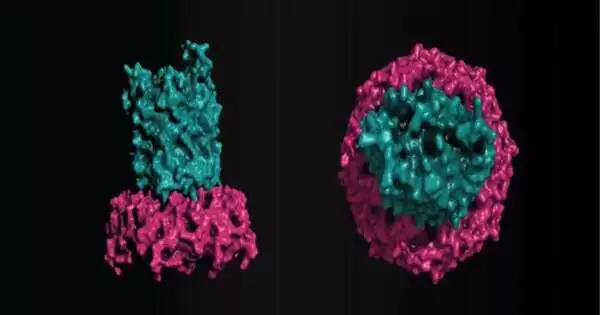
As per current information, EPS would be emitted across the external film by an OPX protein that is coordinated into the layer. However, the group discovered that an external film -barrel protein called EpsX is also important for EPS trade.Then, shockingly, we found a related periplasmic short OPX protein, EpsY, that totally misses the mark on its ability to traverse the external film. Along with Dr. Timo Glatter, we likewise found that EpsX and EpsY straightforwardly connect.
In view of their perceptions and computational primary science, the researchers suggest that EpsX and EpsY address a clever sort of translocon for polysaccharide trade across the external film, where an -barrel protein works expressly as the external layer crossing part in a bipartite complex with a totally periplasmic OPX protein.
As per Lotte Sgaard-Andersen, this definite information could open up better approaches for controlling pathogenic microbes. She makes sense of, “Marco Herfurth, an alumni understudy in my examination bunch, found utilizing computational genomics that comparable composite frameworks are broad in Gram-negative microbes.”
For example, this new framework makes sense of how V. cholerae secretes its VPS polysaccharide, which is significant for biofilm development and harmfulness. Hence, our discoveries not only have huge ramifications for how we might interpret polysaccharide trade in M. xanthus, but in addition, significant ramifications for how we might interpret polysaccharide trade in everyday Gram-negative microbes. “
More information: Johannes Schwabe et al, Evidence for a Widespread Third System for Bacterial Polysaccharide Export across the Outer Membrane Comprising a Composite OPX/β-Barrel Translocon, mBio (2022). DOI: 10.1128/mbio.02032-22
Journal information: mBio