Delivering the undetectable apparent is among researchers’ #1 difficulties.
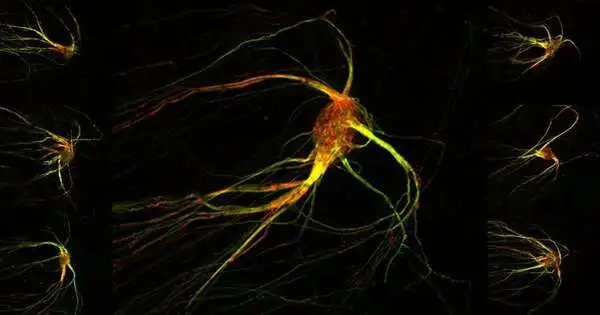
The ocean animal like pictures are tiny representations of synapses that figure out, in any case, undetectable scents, like the smell of a rose or the odor of a spoiled egg. The smooth red and green streaks uncover cells in a mouse’s mind’s smell place: its olfactory bulb. The olfactory bulb is organized into hundreds, even thousands, of isolated groups called glomeruli, and every glomerulus responds with a certain goal in mind to the great many odors of synthetics drifting in the air. The picture above shows a solitary glomerulus where signal-conveying projections (axons) from tactile cells in the nose have met.
Every glomerulus gets signals from its own subset of olfactory neurons, which are arbitrarily conveyed in a creature’s nose yet undeniably tuned to identify smells in the same way. Since the 1990s, scientists have realized that every one of these subsets of olfactory neurons bears a remarkably formed receptor protein (because of a randomizing quality-based process) that locks explicitly onto an alternate scent particle.
Also, that presents a remarkable neuroscientific secret: how might each haphazardly found, scent-identifying cell in the nose figure out how to convey messages to only one explicit glomerulus inside the olfactory bulb? This accomplishment is similar to, say, 50 companions who are initially isolated in irregular spots in a city advancing toward a similar loft without first having the location. Some way or another, they all innately know where to go.
A vital insight into how the olfactory framework accomplishes its wiring accuracy has all the earmarks of being close by. In a review distributed today in Cell, Zuckerman Foundation Head Examiner Stavros Lomvardas, Ph.D., and MD-Ph.D. competitor Hani Shayya led a group that coaxed out what they suspect is the focal getting sorted out process in mice between the nose’s tactile cells and their glomeruli focuses in the mind’s olfactory bulb.
The core of their disclosure lives looking like every receptor protein as it accepts that its novel 3D structure is inside a rounded part of the cell known as the endoplasmic reticulum (trama center). Every protein’s not set in stone by the novel grouping of its amino corrosive parts.
“This technique discovered a genetically encoded, hard-wired method of translating a randomly selected receptor identification to a very precise target in the olfactory bulb.”
Dr. Lomvardas, also a professor of neuroscience and of biochemistry and molecular biophysics
The scientists found that every one of these amino corrosive groupings, the scientists found, forces a quantifiable level of weight on the trama center (envision stuffing different items into a sock). In ways not yet known, these various levels of trama center pressure behave like a dial setting.
Each setting triggers a quality coordinated process by which the tactile cells really direct their axons (through examples of “direction particles”) to their objective glomeruli inside the olfactory bulb. Along these lines, every subset of tactile cells with the equivalent molded receptor protein winds up extending its axons to exactly the same glomerulus. Without a receptor-glomerulus plan of this sort, a rose could wind up resembling a spoiled egg as well as the other way around.
“It is amazing,” said Dr. Lomvardas, likewise a teacher of neuroscience and of natural chemistry and sub-atomic biophysics at Columbia’s Vagelos School of Doctors and Specialists. “This framework figured out how to make a hereditarily encoded, permanently set up method for changing haphazardly picked receptor character to an exact objective in the olfactory bulb.”
He brought up that neurodegenerative illnesses, including Alzheimer’s and Parkinson’s, frequently include olfactory shortages from the get-go in the sickness cycle. This proposes that the early location of disturbances in the olfactory framework’s high-accuracy wiring could turn out to be “clinically significant,” he said.
Shayya highlighted another tempting opportunity. Maybe olfactory neurons are in good company in the manner in which trama center pressure arranges their wiring with downstream neurons. “Assuming, incidentally, all neurons do this, this disclosure could assist us with seeing considerably more about the mind,” said Shayya.
More information: Hani J. Shayya et al, ER stress transforms random olfactory receptor choice into axon targeting precision, Cell (2022). DOI: 10.1016/j.cell.2022.08.025