Teacher Huang Hanmin’s exploration group from the College of Science and Innovation of China (USTC) of the Chinese Foundation of Sciences (CAS) proposed another worldview of metal electron-transport catalysis, which has been pioneeringly utilized to accomplish alkylative aminomethylation of unactivated alkene. Their work was published in Nature Catalysis on August 21.
Progress in metal-catalyzed carbon bond arrangement has been an essential piece of contemporary natural science research. Nonetheless, the advancement of metal-catalyzed C(sp3)-C(sp3) bond development has been moderately delayed because of the age of unsteady alkyl-metal intermediates during the response, which leads to different side responses, making it challenging to apply the old-style reactant worldview to the C(sp3)-C(sp3) security arrangement.
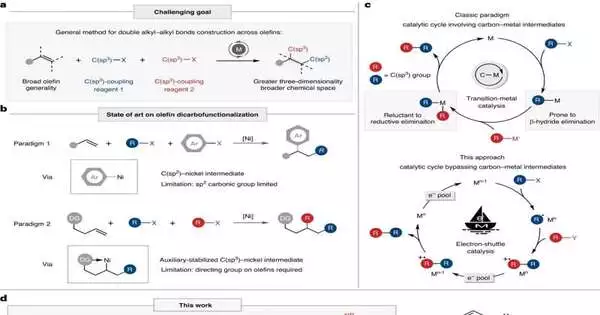
The dicarbofunctionalization of alkenes, where two carbon practical gatherings are presented at the two closures of the alkene in a solitary activity, has acquired consideration for its capacity to productively associate carbon particles, in this way working with the development of complicated particles. Existing examinations are often restricted to the presentation of C(sp2) utilitarian gatherings, or they require the pre-establishment of planning bunches onto the alkenes to settle the alkyl-metal intermediates. This obviously lessens the substrate relevance and step economy of the response.
To tackle the difficulties of alkene difunctionalization responses, the examination group proposed another worldview of metal electron-transport catalysis. In particular, they used a metal impetus as an electron transport to start and extinguish revolutionaries, in this manner accomplishing the development of numerous alkyl securities through revolutionary unsaturated security expansion, successfully staying away from the age of unsteady alkyl-metal intermediates.
In this review, the group utilized a nickel impetus as the electron transport and utilized N, O-acetals, and alkyl halides as the alkylating specialists to achieve the dialkylation of unactivated alkenes. This strategy exhibited astounding similarity with straightforward alkenes, unactivated alkenes, and polysubstituted alkenes.
Also, different kinds of alkylating specialists could be applied under such response conditions. Auxiliary amines and paraformaldehyde could likewise supplant N,O-acetals in the four-part response, further widening the extent of the response’s applications.
This response offers a productive technique for the amalgamation of fluorinated and non-fluorinated δ-amino acids and other unnatural amino-corrosive subsidiaries. In addition, this response presents different useful gatherings, which could additionally change to deliver more important complex atoms. For example, by decreasing and cyclizing the response items, one can quickly develop piperidine compounds, which are pervasive in drug atoms. Analysts combined the drug atom Mepazine and its comparing fluorinated subordinates through this change procedure, exhibiting the usefulness of the response.
This response offers huge engineered relevance as well as fills in as a model of the metal electron-transport catalysis worldview, displaying the promising possibilities of this synergist approach. Further examination of metal electron-transport catalysis will play a significant role in the improvement of medication and utilitarian particle combinations.
More information: Changqing Rao et al, Double alkyl–alkyl bond construction across alkenes enabled by nickel electron-shuttle catalysis, Nature Catalysis (2023). DOI: 10.1038/s41929-023-01015-1