Since the outcome of the coronavirus antibody, RNA treatments have been the object of expanding interest in the biotech world. These treatments work with your body to focus on the hereditary basis of illnesses and diseases, a promising elective treatment strategy compared to that of conventional drug therapy.
Lipid nanoparticles (LNPs) have been effectively utilized in drug conveyance for quite some time. FDA-endorsed treatments use them as vehicles for conveying messenger RNA (mRNA), which prompts the cell to make new proteins, and little meddling RNA (siRNA), which trains the cell to quiet or hinder the outflow of specific proteins.
The greatest test in fostering a fruitful RNA treatment is its designated conveyance. Research is presently facing the flow limits of LNPs, which have left numerous illnesses without a viable RNA treatment.
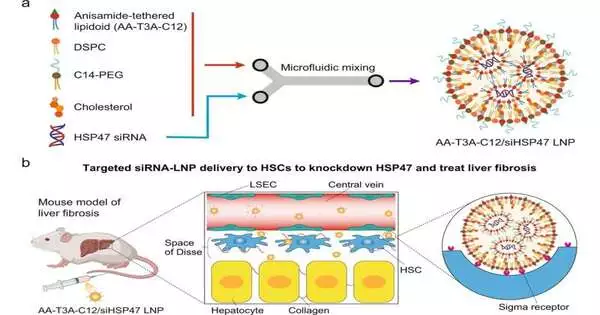
Liver fibrosis occurs when the liver is overly harmed and the healing system causes scar tissue to accumulate, obstructing the liver’s solid functionality.It is an ongoing illness described by the development of an extremely collagen-rich extracellular grid (ECG). Due to a lack of frameworks for focusing on activated liver-occupant fibroblasts, it has been difficult to get involved with RNA treatments in liver fibrosis.Both the strong fibroblast structure and the absence of explicitness or a preference to focus on these fibroblasts have blocked current LNPs from entering enacted liver-occupant fibroblasts, and hence they can’t convey RNA therapeutics.
“We added an anisamide ligand, a chemical with a high affinity for the receptor on these stellate cells, into the structure of the ionizable lipid to produce LNPs specific enough to target hepatic stellate cells, which are responsible for fibrosis. To target and unlock distribution to these difficult-to-reach cells, we developed a lock-and-key mechanism.”
Michael Mitchell
To handle this issue and assist with giving a therapy to the large numbers of individuals who experience the ill effects of this ongoing sickness, Michael Mitchell, J. Peter and Geri Skirkanich, Partner Teacher of Development in the Branch of Bioengineering, and postdoctoral colleagues Xuexiang Han and Ningqiang Gong tracked down a better approach to blending ligand-fastened LNPs, expanding their selectivity, and permitting them to target liver fibroblasts.
Humdinger Xue, Margaret Billingsley, Rakan El-Mayta, Sarah J. Shepherd, Mohamad-Gabriel Alameh, and Drew Weissman, Roberts Family Teacher in Antibody Exploration and Head of the Penn Foundation for RNA Advancement at the Perelman Institute of Medication, likewise added to this work.
Their review, distributed in Nature Correspondences, shows how a little particle ligand integrated into the union of the ionizable lipid, a vital part of the LNP, takes a liking to the famously difficult-to-target enacted fibroblasts in the liver responsible for the development of collagen.
The collagen development is joined by an expanded articulation of Intensity Shock Protein 47 (HSP47), the protein that drives collagen biogenesis and emission. HSP47 overexpression and increased collagen biogenesis in advanced fibrosis.
When their LNPs show up at and enter the objective cell, siRNA is delivered, which quiets the outflow of HSP47, hinders the creation of collagen, and leaves fibrosis speechless. The treatment, demonstrated to be successful in mice, is a promising treatment for liver fibrosis in people.
This clever approach to ionizable lipid union opens the door to many more avenues for RNA treatment to treat various diseases.
“To make LNPs sufficiently specific to target hepatic stellate cells, those that drive fibrosis, we integrated an anisamide ligand, a particle that has a high liking for the receptor on these stellate cells, into the design of the ionizable lipid,” says Mitchell. “Basically, we made a lock-and-key system to target and open conveyance to these hard-to-arrive-at cells.”
The union cycle was created by Han and partners as a “one-pot, two-step” process. To make a library of ionizable lipids, the group initially put an anisamide ligand (AA) forerunner and different amino centers together. They then added the hydrophobic tail to make AA-fastened ionizable lipids. Anisamide was picked as the ligand because of its unbiased and stable nature, as well as its affinity for the overexpressed sigma receptors on stellate cells. After creating a library of AA-fastened LNPs, the researchers tested their ability to target and deliver treatment to cells using a two-round selection cycle.
“We expected to find a particular AA-fastened LNP that was both strong and specific,” says Han. “The main round of the choice cycle was finished by analyzing how well our LNPs could thump down green fluorescence protein (GFP) in fibroblasts to gauge power.” “GFP gives extraordinary visual proof of how helpful RNA switches off quality articulation continuously.”
“In the subsequent round, we tried the specific capacity of the strong LNP,” says Han. “We did this by impeding the sigma receptor to comprehend how huge the particular AA ligand bunch was in the LNP’s capacity to get into target cells. “Obviously, we showed that the AA bunch was huge; after the sigma receptor bar, we lost the lock-and-key system, and the AA-fastened LNP wouldn’t enter the objective cell.”
The researchers identified AA-T3A-C12 as a powerful and specific LNP carrying beneficial siRNA capable of achieving 65% knockdown of HSP47 articulation in mice as well as improving the recovery of damaged liver tissue.The consequences of the review show that the AA-T3A-C12 LNP beats the MC3 LNP, a clinically used non-viral vector that has been FDA-endorsed for use in hepatic, or liver, cell RNA treatment.
This new ligand-fastened LNP gives a type of treatment for liver fibrosis, and the union strategy gives a method for fitting LNPs to other previously difficult-to-target cells and tissues in the body.
“The capability of LNPs is huge,” says Han. “We’re making LNPs more astute and effective.”
“We are eager to have created a potential treatment that handles the hereditary base of this liver illness,” says Mitchell. “What’s more, since this LNP conveyance vehicle works in fibrotic cells of the liver, it might prompt the development of treatments for different kinds of fibrosis in the body, for example, fibrosis that emerges in the lung or in cancers.”
“Past what we have examined in the liver, this strategy for making LNPs can be utilized to open the door for treatments in other cell types,” he adds. “We might actually target cells in the mind, lungs, or heart by introducing explicit ligands into the ionizable lipid structure. There are numerous roads from here, and we are eager to keep pushing this exploration in new directions.”
More information: Xuexiang Han et al, Ligand-tethered lipid nanoparticles for targeted RNA delivery to treat liver fibrosis, Nature Communications (2023). DOI: 10.1038/s41467-022-35637-z
Journal information: Nature Communications