In a “self-organized” environment, the genetic material (DNA) and proteins of the living cell contain physiologically relevant components. The underlying mechanism of living matter’s self-organization can be revealed by comprehending this self-assembling process.
Droplets of water and oil (w/o) or water and water (w/w) can be used to study cellular self-assembly as prototypes or “models” that mimic cells. Biomedical research will also greatly benefit from these models. Although complicated and expensive equipment can be used to create cell mimics, the associated methods are costly, time-consuming, and difficult.
Now, “microgels”—uniform gelatin-based cell mimics—have recently been created by Japanese researchers in a one-step process. On May 24, 2023, the associated findings appeared in the journal Small.
“Right now, our research is focused on understanding the self-organization of living matter. As a result of our investigation, we discovered an experimental approach that may be highly useful for the creation of microgels.”
Gen Honda and Miho Yanagisawa of The University of Tokyo,
MS, please explain the reason for their study. The study’s principal investigators, Mayu Shono and Prof. Akihisa Shioi of Doshisha University, stated, “Our research focuses on understanding the self-organization of living matter at the moment.” We have discovered an experimental method that may be quite useful for the production of microgels as an extension of our research. Gen Honda and Miho Yanagisawa from The University of Tokyo, in addition to Kenichi Yoshikawa from Doshisha University and Kyoto University, were members of the research team.
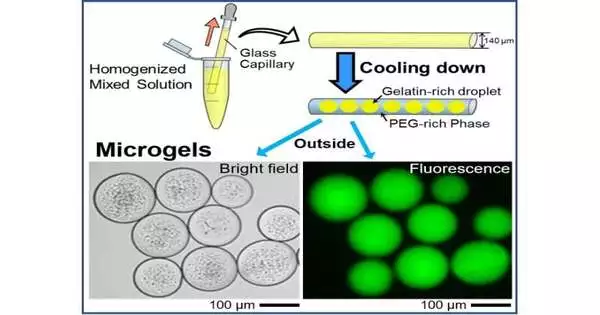
Indeed, the process by which microgels are formed is intriguing. The first step is to make domain structures out of gelatin and polyethylene glycol (PEG), two common synthetic crosslinkers. The selective transition of the gelatin-rich domain into the gel phase is favored when the temperature is decreased to 24 °C.
The PEG-rich phase preferentially migrates to the glass surface of the capillary tube under a specific set of experimental conditions because it has a higher affinity for glass than it does for the gelatin-rich domains. Consequently, the PEG-rich phase takes over the gelatin-rich droplets. Glass capillary experiments were used in theoretical and numerical modeling studies to confirm these results, which showed that w/w phase separation was dominated by the wettability of the inner surface of the glass capillary.
Due to the phase separation of PEG and gelatin, the gelatin-rich droplets were also able to spontaneously entrap DNA molecules when DNA was added, resulting in cell-like microgels. Additionally, the study found that the droplets’ inclusion of negatively charged DNA molecules could prevent their fusion even at temperatures above the sol-gel transition.
The encapsulated DNA was also labeled and tracked by the team using a fluorescent dye. The glowing DNA molecules were found to be housed in round microgel structures during subsequent fluorescence microscopy tests. The current method is expected to confine, store, and transport enormous DNA molecules within tiny droplets the size of cells, according to the authors.
“This novel method to form uniform cell-sized microgels may be applicable to other biopolymers,” says Ph.D. student Mayu Shono, the first author, who is excited about the scope of their research going forward. In addition, the uniformly sized and stable cell-like systems will have significant repercussions for the biological and life sciences.”
In a nutshell, the study discusses a novel method for the preparation of gelatin-based cell mimics that can be adapted to suit the application’s needs. According to Prof. Shioi’s conclusion, “the method proposed in our study may be useful for producing microgels for food, medicines, cosmetics, and other materials,” as it does not require any special equipment, organic solvents, or surfactants.
More information: Mayu Shono et al, Spontaneous Formation of Uniform Cell‐Sized Microgels through Water/Water Phase Separation, Small (2023). DOI: 10.1002/smll.202302193