The development of electrons across wires permits us to utilize power consistently. Natural nanowires, minuscule wires made of proteins, definitely stand out enough to be noticed for their capacity to convey electrons over significant distances.
In a review distributed in Little by the Vermaas lab at the MSU-DOE Plant Exploration Lab, specialists explore how we might interpret organic nanowires using programmatic experiences.
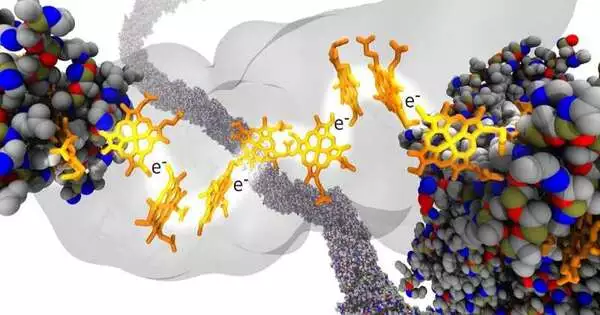
Martin Kulke, the first creator of the review, joined by the Vermaas lab group, made reenactments of precious stones involving information from the genuine examinations in the PRL Kramer lab, where they pointed a light source at a nanocrystal comprised of proteins and determined how quickly energized electrons went through it. The genuine inquiry was the reason electron movement was getting slower with expanding temperature, which as a rule speeds up processes at the nanoscale.
“To put this theory to the test, we simulated these protein nanocrystals at various temperatures. We discovered that the distance changes between temperatures are not as dramatic on their own.”
said Josh Vermaas, primary investigator for this study and assistant professor in the Department of Biochemistry and Molecular Biology and at the PRL.
One potential thought was that the distance electrons would have to hop inside the nanocrystal could increment with temperature, dialing back how quick they could travel through the protein.
“We reenacted these protein nanocrystals at various temperatures to test this thought,” said Josh Vermaas, essential agent for this review and right-hand teacher in the Division of Natural Chemistry and Sub-atomic Science and at the PRL. “What we found is that the distance changes across various temperatures are not really emotional all by themselves.”
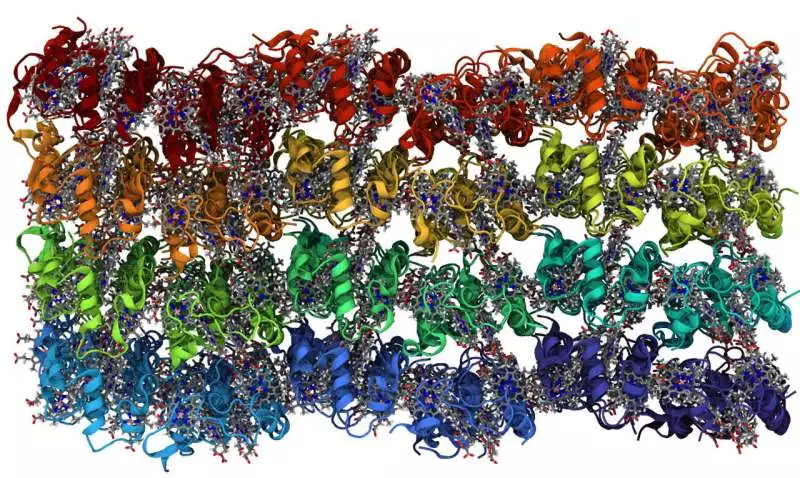
In this portrayal, every one of the 96 proteins in the nanocrystal is an alternate tone. The electrons go from gathering to bunching inside the protein. The hemes are displayed in a stick portrayal, with dark for the carbons, blue for the nitrogens and a pink iron molecule. Credit: Vermaas Lab
At the point when factors other than temperature were controlled, the analysts began to see some fascinating activity from the electrons’ bounces inside the nanowire. The nanowire protein network was made longer, more limited, thicker, and more slender to distinguish bottlenecks to the electron stream inside the nanocrystal.
“We found that in natural nanowires, the electron transport depends on the movement of the proteins in the wire,” Kulke said. “This means eventually, the more you make those nanowires, the less electron transport you overcome, and the thicker you make them, the more electron transport you get past them.”
The utilization of natural nanowires is speculative right now, yet understanding how they can be built to take into consideration more electron streams is pivotal to future endeavors utilizing them to interface organic cycles with traditional hardware.
More information: Martin Kulke et al. Long-Range Electron Transport Rates Depend on Wire Dimensions in Cytochrome Nanowires, Small (2023). DOI: 10.1002/smll.202304013