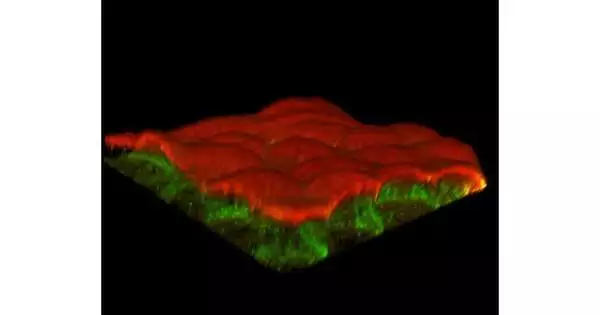
Minuscule things matter—for example, one aminocorrosive can totally modify the design of the cell. Specialists at the Colleges of Göttingen and Warwick examined the construction and mechanics of the principal part of the cell’s cytoskeleton, a protein known as actin. Actin is tracked down in every single living cell, with a scope of significant capabilities ranging from muscle constriction to cell flagging and shape.
This protein comes in two assortments named “isoforms,” known as gamma-actin and beta-actin. The distinction between the two proteins is microscopic—a couple of amino acids at only one piece of the particle shift. However, this little change hugely affects the cell. In nature, typically, just combinations of the two isoforms are found. In their review, the analysts isolated the two isoforms and examined them exclusively. The outcomes were distributed in the diary, Nature Correspondences.
The scientists concentrated on the way of behaving of organizations of fibers, especially zeroing in on the extraordinary properties of the individual isoforms. They utilized particular strategies permitting them to survey the mechanics and elements of examination models of cytoskeletal networks, focusing on mastery biophysics at Göttingen and bioengineering at Warwick.
“Our findings are compelling because they open up new avenues for understanding the complex dynamics of protein networks within cells.”
Professor Andreas Janshoff, Institute for Physical Chemistry, University of Göttingen.
The outcomes demonstrate that gamma-actin likes to shape unbending organizations close to the cell’s peak, while beta-actin specially frames equal packs with a particular hierarchical example. This distinction is probably going to be because of the more grounded cooperation of gamma-actin with explicit kinds of emphatically charged particles, which makes its organizations stiffer than those framed by beta-actin.
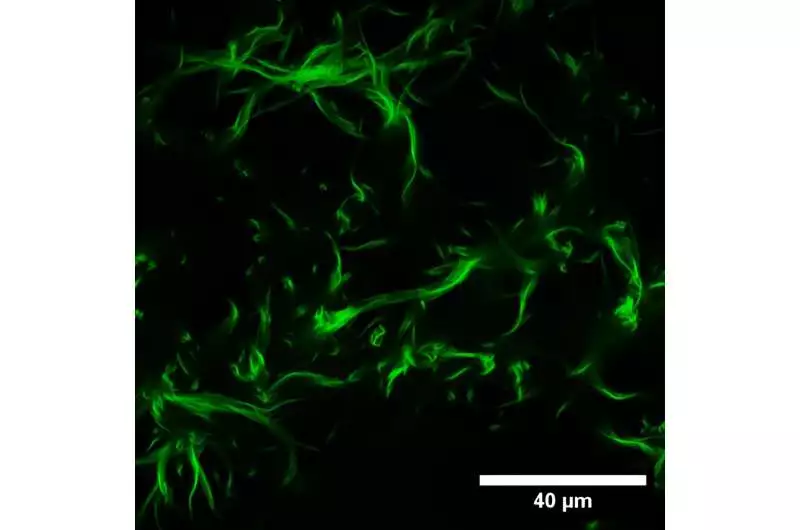
Magnifying lens picture showing the beginning phase of actin packaging within the sight of magnesium particles. Credit: Andreas Janshoff
“Our discoveries are convincing on the grounds that they open up new roads for understanding the mind-boggling elements of protein networks inside cells,” says Teacher Andreas Janshoff, Organization for Actual Science, College of Göttingen.
The exploration progresses’ comprehension researchers might interpret crucial cell processes by revealing insight into explicit natural elements of actin, and this will have specific significance for processes including cell mechanics like development, division, and development of cells in tissue.
“The ramifications of these disclosures reach out to the more extensive field of cell science, offering bits of knowledge that could affect numerous areas of exploration and applications, for example, in formative science,” adds Janshoff.
More information: Peter Nietmann et al. Cytosolic actin isoforms form networks with different rheological properties that indicate specific biological functions, Nature Communications (2023). DOI: 10.1038/s41467-023-43653-w