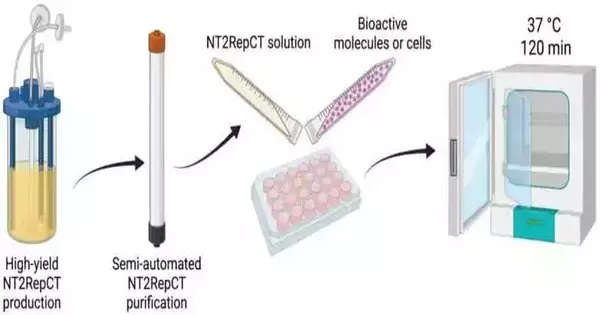
Recombinant spider silk protein hydrogels have a lot going for them, according to a new study from the Karolinska Institutet. By simply incubating at 37°C, they enable the encapsulation of cells and bioactive molecules. The fibrillar network imitates that of the extracellular matrix, and their transparency makes it possible to observe encased cells. These hydrogels can be utilized for continuous drug release because their mechanical properties are comparable to those of various tissues.
Artificial spider silk is a promising biomaterial if it is produced from proteins produced in heterologous hosts. A biomimetic production method for spider silk, developed by researchers at Karolinska Institutet’s Department of Biosciences and Nutrition, enables the production of artificial spider silk proteins and fibers without the use of harsh organic solvents.
This was made conceivable by the use of a little insect silk protein (spidroin). This mini-spidroin has a significant advantage in that it can be purified semiautomatically and produced in large quantities (14.5g of purified protein per liter of culture in bioreactors).
The team recently discovered that, when incubated at 37°C, recombinant mini-spidroins quickly form hydrogels without the use of crosslinkers. The gels are made up of a nano-sized fibrillar network, making it possible to create a novel hydrogel system for cell culture and tissue engineering. The gels form at temperatures and times that are compatible with most living cells and bioactive agents.
Tunable to match various tissues, the new study by researchers Tina Arndt and Urmimala Chatterjee, which was published in Advanced Functional Materials, demonstrates that human fetal mesenchymal stem cells are capable of being encapsulated in these hydrogels and exhibiting high survival and viability afterward. It is interesting to note that the hydrogels’ mechanical properties can be altered by adjusting the protein concentration to match those of various tissues, such as muscles and cartilage.
Moreover, combinations of recombinant bug silk proteins and green fluorescent protein (GFP) structure gels, from which practical GFP is continuously delivered, showing that bioactive atoms, effectively remembered for the gels, keep up with their movement and can diffuse through the gel. This implies that the hydrogels have expected applications in drug conveyance.
Along a similar line, epitomized ARPE-19 cells are feasible and constantly produce the development factor progranulin, which is set free from the cells, diffuses through the hydrogel, and is distinguished in the cell culture medium over the review time of 31 days.
More information: Tina Arndt et al, Tuneable Recombinant Spider Silk Protein Hydrogels for Drug Release and 3D Cell Culture, Advanced Functional Materials (2023). DOI: 10.1002/adfm.202303622