Mixing can permit the equal scattering of substances in fluids. Einstein’s tea leaf oddity is an idea that shows how tea leaves can pack in a donut shape through an optional stream impact during blending. In another review distributed in Science Advances, Zehui Zhang and partners in physical science and design in China showed that Einstein’s tea leaf conundrum (contracted as ETLP) prompted a focus on nanofluids.
They achieved this by reproducing the nanoparticle direction under blending to get a grayscale investigation of nanofluids under mixing and standing cycles. The group applied the confined focus to accomplish ultrafast accumulation of gold nanoparticles to shape gold aerogels. They changed the gold aerogels from around 10 to 200 nm and fostered a constituent of very high virtue and crystallinity to uncover possible applications in photocatalysis and surface-upgraded Raman dispersing.
Einstein’s tea leaf oddity
In 1926, Albert Einstein depicted a straightforward exploratory perception while blending tea, where the leaves followed a twisting direction towards the focal point of the cup. In like manner, the social event of tea passes on under mixing because the optional stream is helpful to gather microscale particles in scattering frameworks. Since nanoparticles with better steadiness as a rule move along with the liquid because of Brownian movement, during Einstein’s tea leaf mystery, the stream speed conundrum initiated laminar streams, driving the limited fixation or collection of colloidal nanoparticles inside the slender stream.
Materials researchers have zeroed in on metal aerogels like gold in catalysis, retention, and gadget biocompatibility applications, as well as in electrochemistry. Regularly, three primary courses can be utilized to plan metal aerogels. In this work, Zhang and associates showed the restricted accumulation of gold nanoparticles and the guidelines for the microstructures of gold aerogels. Einstein’s tea leaf oddity initiated a limited accumulation of metal particles that prepared different sorts of gels, or aerogels.
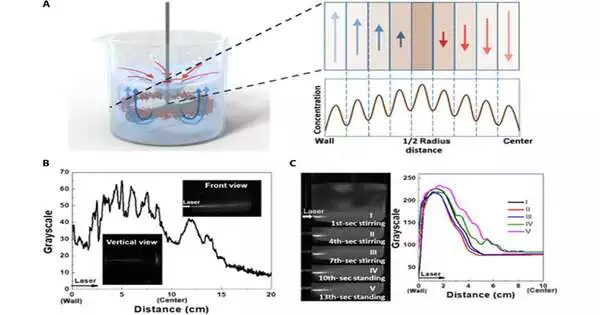
Speculative model and test exhibition of ETLP (A) Schematic chart of NP dissemination under ETLP impact On the right side are the alleged sectional perspectives on laminar streams and the circulation of NPs in the left 50% of a measuring utensil. (B) The grayscale bend (vertical view), front-view photograph, and vertical-view photograph of SiO2 scattering while at the same time mixing. (C) The photographs (left) and relating dim scale (right) of the SiO2 scattering from the front view Five photographs were taken persistently every 3 seconds, while blending was begun toward the start and halted at the eighth second. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi9108
Showing the convention in the nanofield
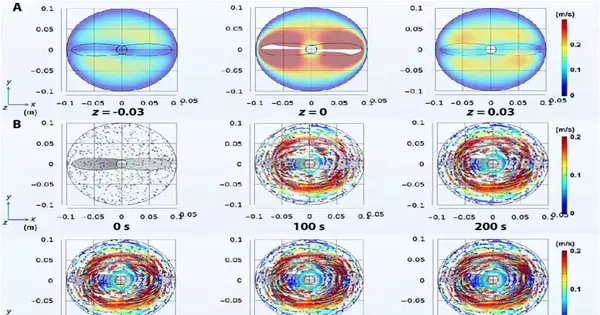
The researchers concentrated on the connection between nanoparticle dissemination and stream speed in nanofluids by utilizing COMSOL Multiphysics programming to reproduce the development of nanoparticles in laminar streams under mixing. They observed the nanoparticle direction in the wake of blending for 500 seconds, where the nanoparticles in the center moved quicker in a more extended direction. The high movement recurrence and sufficiency of the nanoparticles in the high-speed areas advanced the experiences of nanoparticles to make them more focused or crosslinked.
In view of the results, Zhang and the group accepted that the movement of nanoparticles in nanofluids would follow the ETLP (Einstein’s Tea Leaf Catch 22) regulation. To exhibit ETLP regulation at the nanoscale, the group scattered the 50-nm circular silicon dioxide nanoparticles in deionized water as a nanofluid. The nanoparticles displayed plainly visible ETLP with restricted focus impacts in nanofluids.
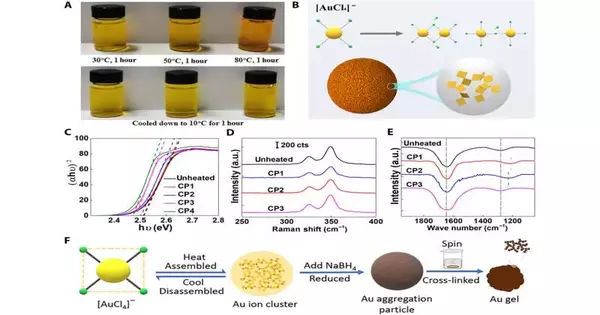
Collect dismantle process in HAuCl4 arrangement. (A) The variety change of HAuCl4 arrangement when warmed and chilled off: HAuCl4 arrangement warmed at 30°, 50°, and 80°C for 60 minutes, individually, and afterward chilled off to 10°C. (B) Assumed system of Au particle group development: [AuCl4]− might be dechlorinated and cochlorinated to shape huge Au particle groups. (C) hν-αhν diagram changed over from fig. S10A (UV-Vis of the HAuCl4 arrangement was estimated from 80°C to room temperature constantly multiple times.) (D) Raman shift of 2.5% HAuCl4 arrangement during warming and cooling processes, a.u., erratic units (E) FTIR spectra of a 10% HAuCl4 arrangement were estimated consistently multiple times from 80°C to room temperature. (F) The entire arrangement process The mix of [AuCl4]− could be utilized to control the skeleton size of GAs. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi9108
Creating vaporous aerogels
The exploration group arranged a privately totaled gold gel by decreasing gold particle bunches through Einstein’s tea leaf Catch 22 interaction. They framed a chloroauric corrosive (HAuCl4) arrangement with the gold bunches and dried the constituents at room temperature or under a warming wellspring of light for transmission electron microscopy perceptions.
Under light warming, the particles accumulated into groups, which the group additionally saw with estimations and investigation. These included the conductivity and pH value of the gold arrangement estimated during the warming and cooling processes. By directing the temperature of the forerunner arrangement, the scientists arranged three gold aerogel tests through mixing in 20 minutes or less. In any case, without blending, there was no undeniable gel development in the gold arrangement, even after 24 hours and at 80°C.
Portrayal and utilization of gold nanoparticles
Zhang and associates examined the skeleton microstructure of the aerogels by utilizing little-point X-beam dispersing, checking electron microscopy, and transmission electron microscopy. The size of the gold particles in the aerogel was eminently unique.
Utilizing X-beam photoelectron spectroscopy, the researchers recognized the natural creation of three examples. Beside carbon from a wellspring of pollution, they noticed just gold in the organization of the aerogels. The readiness interaction made some critical memories save quality, shaping gold aerogels with a huge scope of microstructure sizes and high virtue.
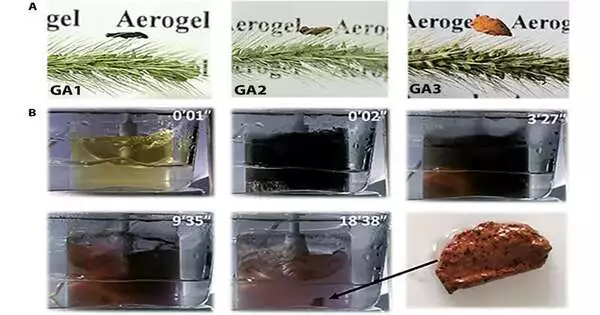
Photos and readiness cycle of GAs (A) Photographs of GAs (B) ETLP-prompted conglomeration of GA3: scattered HAuCl4 arrangement, HAuCl4 arrangement subsequent to adding the hesitance, earthy colored particles encouraged in the sol, a little gel collected from earthy colored particles, the developed gel with bigger size, while the shade of arrangement turned light eminently, and the Au gel got. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi9108
Standpoint
Along these lines, Zehui Zhang and his group affirmed Einstein’s blue-green leaf mystery (ETLP) as a material for nanofluids with a suddenly confined conglomeration impact to shape gold aerogels by just mixing.
The researchers developed gold particle groups of various sizes by managing the temperature of chloroauric corrosive. They finished the examinations with ETLP-driven collection impacts and carbon dioxide drying to create aerogels with differing skeleton sizes, with a limit with regards to future aerogels being arranged much the same way.
More information: Zehui Zhang et al, Einstein’s tea leaf paradox induced localized aggregation of nanoparticles and their conversion to gold aerogels, Science Advances (2023). DOI: 10.1126/sciadv.adi9108