Materials researchers have widely concentrated on quick particle pervasion in nanofluidic diverts for a long time because of their true capacity inside filtration advancements and osmotic energy collection. While the components hidden particle transport still can’t seem to be perceived, the cycle can be accomplished in nanochannels created in a painstakingly controlled way.
In another report presently distributed in Science Advances, Yu Jiang and an exploration group in the actual science of strong surfaces in China depicted the improvement of two-layered nanochannels with their top and base walls containing molecularly level graphite and mica precious stones.
The unmistakable wall designs and properties permitted the examination of associations among particles and inside surfaces. The group noted upgraded particle transport inside the channels that is significantly quicker than in mass arrangements, giving insights into surface impacts on particle transport at the nanoscale.
Nanoscale particle transport
Systems of nanoscale particle transport can outflank their macroscale partners because of their vehicle rates. Models incorporate quick particle course through protein diverts in cell layers in a cycle that is basic for the fundamental workings of life. These incorporate particle saturation through nanoporous films for water cleaning, particle division, and osmotic power generation. To comprehend the components of quick particle transport at the nanoscale, scientists should make nanochannels with all-around directed math and inside structures.
Yu Jiang and his group explored the beginning of quick ionic vehicles inside nanochannels containing particle adsorption destinations on the inside. The improved plan limited the possibility of tainting channel insides with synthetics and polymers during creation to concentrate on adsorption impacts on perfect surfaces.
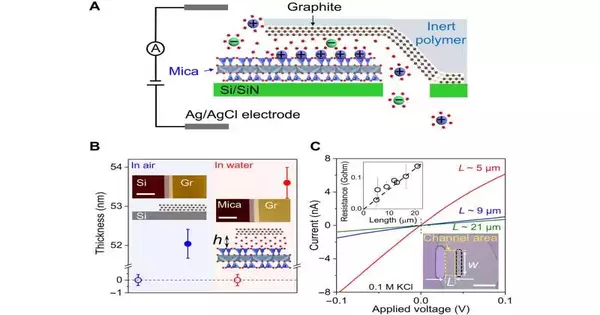
During the examinations, Jiang and associates gathered precisely shed graphite and mica gems and moved them to an opening on silicon substrates. They adjusted the graphite/mica heterostructures with the gap for the top graphite layer cover, while the base layer lined up with the gap at their not entirely set in stone by the exchange strategy.
The researchers utilized a nuclear power magnifying lens to quantify the thickness of the top graphite on mica in fluid arrangements. They then estimated the mean level of mica and graphite surfaces in the channel locale. Since graphite and mica layers can delaminate at high salt centralizations of 2 M with somewhat enormous ionic flows through the channels, they utilized arrangements with salt fixations equivalent to or more modest than 0.1 M for trial precision.
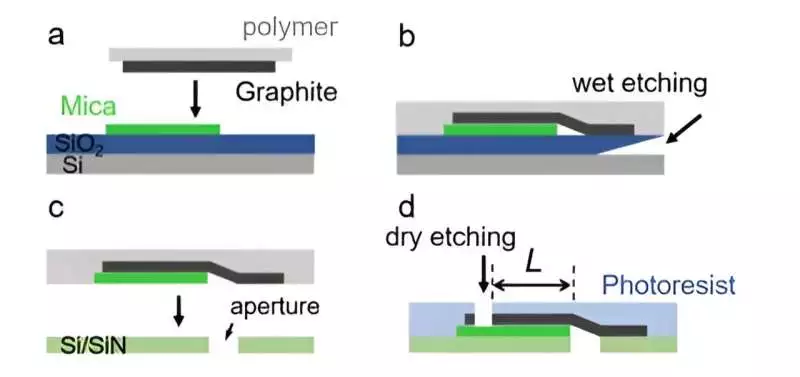
Gadget creation and portrayal creation of a stream of graphite-mica channels (a) A graphite piece is moved onto mica through a dry exchange procedure. (b) and (c) The graphite-mica stack is moved onto the opening utilizing the wet exchange method. (d) Channel length is characterized by dry scratching techniques. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adi8493
Extra tests
The researchers assessed the powerful level of the channels seen by particles and affirmed the level portrayed by nuclear power microscopy. During the examinations, they filled the two repositories with different chloride arrangements of 0.1 M and 0.01 M focuses, separately, to make a fixation slope.
Jiang and partners concentrated on the superficial-level impacts of the channel’s inside upon particle transport and estimated the ionic conductivity of potassium chloride as a component of its mass fixation. The group explored the particle transport process in the G-mica channels and limited the quantity of potential components by playing out extra estimations.
Standpoint
The high conductance and particular particle adsorption on mica surfaces demonstrated extensive surface dispersion. The researchers presented a quantitative articulation for particle transport in the graphite-mica channels to give experience to related components.
They depicted the surface conductivity to be because of the movement of adsorbed cations while thinking about the compelling surface salt number thickness and the surface versatility of adsorbed cations and zeroed in on the vehicle of monovalent cations. The generally enormous adsorption energy of cations restricted their desorption before movement, which highlighted the significance of mica for particle transport.
Along these lines, Yu Jiang and colleagues featured surface dispersion as an extra particle transport method in nanofluidics to give ionic conductivity that is significantly higher than in mass arrangements. The value is among the most elevated from single nanochannels. The ability to make channels utilizing mica bunch gems that have inclinations toward adsorbing assorted cations can recognize particles that rely upon their adsorption energies for particle transport and detection applications.
More information: Yu Jiang et al, Surface diffusion enhanced ion transport through two-dimensional nanochannels, Science Advances (2023). DOI: 10.1126/sciadv.adi8493