protein capability and is not set in stone by both their gathering and optional design. Anomalies connected with either protein collection or optional design can prompt neurodegenerative illnesses. In another review, a global exploration group reveals how fluoride nanoparticles, materials utilized in vivo imaging, influence the gathering and design of the amyloid beta protein. Their outcomes present a stage towards better treatment and counteraction of neurologic issues like Alzheimer’s illness.
Self-gathering, or the relationship of individual units of a material into requested designs or examples, is a peculiarity of incredible research interest for materials researchers. One noticeable illustration of self-gathering comes from the self-gathering of proteins in natural frameworks. The capability and action of proteins are represented by their gathering state. Moreover, the protein’s “optional design,” described by its collapsing into structures like -sheets, likewise assumes a part. As a matter of fact, irregularities in the protein optional designs or their gathering can prompt different neurodegenerative illnesses, including Alzheimer’s sickness.
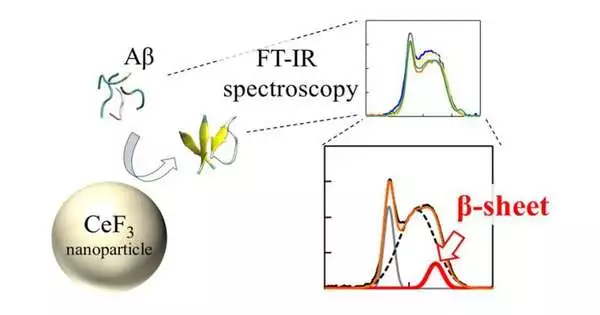
“We discovered that peptides are more likely to form -sheets near the nanoparticle surface. This is a result of hydrophobicity. Parts of the peptide that are rejected by the water solution attach to the nanoparticles more easily and form aggregates.”
Dr. Umezawa
Nanoparticles (NPs) offer a promising path for the treatment and prevention of such illnesses by allowing for controlled and targeted drug delivery.Also, inorganic NPs, like fluoride NPs, are utilized in mind imaging applications. Compared with natural NPs, inorganic NPs are viewed as superior contenders for growing highly useful materials. Yet, there is a lot of concern in regards to their biotoxicity. While their connections with bioproteins have been examined, the system-basic nature of these associations is not yet known.
A global group of researchers from Tokyo University of Science (TUS) in Japan and Nazarbayev University in Kazakhstan has now resolved this issue. In their review, which was made accessible web-based on June 2, 2022 and was distributed in the journal ACS Applied Bio Materials on June 20, 2022, the group explored a part of the amyloid peptide (a protein found in the plaques framing the minds of patients with Alzheimer’s illness) in an answer with fluoride clay (CeF3) NPs. The review was driven by Junior Associate Professor Masakazu Umezawa and included commitments from Mr. Naoya Sakaguchi from TUS and Assistant Professors Mehdi Amouei Torkmahalleh and Dhawal Shah from Nazarbayev University.
There are recreation previews of the peptide total connections with different particles. Recreation aftereffects of the impact of particles within 0.1 nm on the peptides in the frameworks under study: No salt (a), 0.15 M NaCl (b), 0.15 M NH4Cl (c), and 0.15 M NaNO3 (d).sheet = yellow; Na+ = green; NH4+ = blue and white; Cl-= purple; and NO3 = blue and red. Masakazu Umezawa/Tokyo University of Science and TechnologyLicense type: CC BY 4.0
The group utilized a method called “Fourier change infrared spectroscopy” (FTIR) to straightforwardly screen the impact of the NP surface on the peptide bonds. “We found that close to the nanoparticle surface, peptides are bound to framed -sheets. This is due to the effect of hydrophobicity.” pieces of the peptide that are repulsed by the water arrangement adhere to the nanoparticles, and the structure totals more effectively,” makes sense to Dr. Umezawa.
Also, the group explored the impact of other encompassing particles in the arrangement. “What we found was extremely amazing. Indeed, even without the nanoparticles, the climate impacted the pace of optional design development, “says Dr. Umezawa.” This impact, coming about because of a mix of electrostatic connection and hydrogen holding, was overstated after adding nanoparticles. With a cautious selection of particles and nanoparticles, the -sheet development can be either stifled or advanced. This infers that the cycle can be controlled and designed to kill unfriendly impacts. “
The trial results were supplemented with atomic element recreations performed by the Nazarbayev University group. This, thus, helped plan and guide the tests as well as give experience of the outcomes.
With this more profound understanding of the connection between proteins and NPs, the review lays the foundation for controlled protein collapsing processes. With such control, any protein distortions could be killed, and positive connections and primary changes could be advanced. This could prompt a superior counteraction and treatment convention for Alzheimer’s illness and, at last, lead to a superior personal satisfaction for matured grown-ups.
More information: Naoya Sakaguchi et al, Changes in the Secondary Structure and Assembly of Proteins on Fluoride Ceramic (CeF3) Nanoparticle Surfaces, ACS Applied Bio Materials (2022). DOI: 10.1021/acsabm.2c00239