A new study sheds light on antibody measurements that can assist in predicting the Moderna mRNA vaccine’s COVID-19 protective capabilities.
In order to find potential correlates of protection—CoPs—the measurement of immunological markers that are associated with the level of vaccine protection, such as the quantity of antibodies—the researchers looked at data from a phase 3 clinical trial as well as data from the laboratory. A CoP is an important test that shows how well a particular vaccine protects people from infection. Another way to look at it is that a CoP is a sign in the immune system that, once found, can accurately predict how well a vaccine will protect against infection or severe disease.
There are powerful predictors out there right now, but scientists want even more precision. Thus, the quest for additional conclusive Police.
Because these correlates measure a vaccine’s effectiveness, finding them is crucial. Up to this point, the new review shows that the titer of antibodies possibly sparkles as areas of strength for an of Moderna’s mRNA immunization insurance. A titer is the presence and sum antibodies that can kill an infection; for this situation, SARS-CoV-2.
“A priority in COVID-19 vaccine research is the identification and validation of a correlate of protection, an immune biomarker that can be used to reliably predict the degree of vaccine efficacy against a clinically relevant outcome,”
Dr. David Benkeser of Emory University’s Rollins School of Public Health,
In a paper that was published in Science Translational Medicine, researchers looked at immunological data from the COVE clinical trial as well as data from research that looked at a pseudovirus that looked like SARS-CoV-2. The Coronavirus Efficacy (COVE) phase 3 trial, or COVE study, was launched in July 2020 to evaluate the safety and effectiveness of the Moderna vaccine known as mRNA-1273.
While a free information and wellbeing checking board discovered that the immunization met the pre-determined adequacy measures at the principal break examination, the new exploration bored further into the immunological information to look for associates of security, the obvious mark in the resistant framework.
“The ID and approval of a correspond of security, a safe biomarker that can be utilized to dependably foresee the level of immunization viability against a clinically significant result is vital in Coronavirus antibody research,” composes Dr. David Benkeser of Emory College’s Rollins School of General Wellbeing, and lead creator of the multi-focus review.
“CoPs are useful for speeding up the creation and use of vaccines. For instance, for an immunization with laid out viability, a CoP could act as an essential endpoint for immunobridging of antibody adequacy to an objective populace that was excluded from the randomized preliminary,” Benkeser added.
Immunobridging is a method for clinical trials in which an accepted surrogate measure of efficacy is used to infer a vaccine or drug’s effectiveness. Immunobridging can expedite patient treatment. Therefore, it is essential to locate the biomarker or biomarkers that can assist in the introduction of a vaccine to populations in order to combat a virus that is currently in circulation.
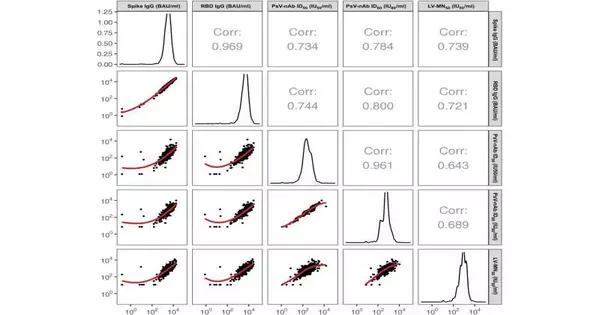
“Normal Police for authorized immunizations are estimations of restricting antibodies — bAbs — or killing antibodies — Catches — and various lines of examination have upheld these insusceptible markers as Police for Coronavirus antibodies.”
The large multi-center team discovered at least one potent biomarker in the study: Across all investigations, proof for corresponds was more grounded for Seizes estimated by the pseudovirus-based versus live infection based balance measure, predictable with the discoveries of a nonhuman primate challenge study,” Benkeser composed.
Benkeser and a few other Emory University team members were joined by collaborators from a number of prominent American research institutions, including Duke University’s Human Vaccine Institute in Durham, North Carolina; the National Institute of Allergy and Infectious Diseases’ Vaccine Research Center in Bethesda, Maryland; the Immunization and Irresistible Illness division of the Fred Hutchinson Malignant growth Community in Seattle, Washington, and Moderna, Inc. in Cambridge, Massachusetts.
According to Benkeser and colleagues, phase 3 trials, such as the COVE study, provide “particularly valuable evidence to support an immune biomarker as a CoP.” The COVE trial involved 30,420 participants who were randomly assigned to receive mRNA-1273 or a placebo in a 1:1 ratio. In the dazed preliminary, immunization viability was 93.2%, Benkeser and colleagues say.
The researchers compared the correlations between vaccine protection and various antibody markers, including a novel one based on live virus neutralization, employing a multivariate model. They found that higher titers of antibodies against SARS-CoV-2’s spike protein and titers of killing antibodies against SARS-CoV-2 pseudovirus were solid corresponds of hazard. In modeling experiments, the strongest independent predictors of vaccine protection were also titers of pseudovirus neutralizing antibodies.
“Future analyses should provide multiple insights relevant for guiding vaccine development and use in the contemporary context of the COVID-19 pandemic,” Benkeser and colleagues concluded. “Given that the correlate analyses of COVE to date have been limited to SARS-CoV-2 nave individuals,” they said. “Future analyses should provide multiple insights relevant for guiding vaccine development and use in the contemporary context of the COVID-19 pandemic.”
More information: David Benkeser et al, Comparing antibody assays as correlates of protection against COVID-19 in the COVE mRNA-1273 vaccine efficacy trial, Science Translational Medicine (2023). DOI: 10.1126/scitranslmed.ade9078