A group of scientists has identified the main impediment of a typical strong state hydrogen material, preparing it for future plan rules and widespread commercial use.
Subtleties of their discoveries were distributed in the Diary of Materials Science A, where the article was highlighted as a title page article.
Hydrogen will play a huge part in driving our future. It’s plentiful and creates no harmful emanations when consumed. In any case, the capacity and transportation of hydrogen are both expensive and dangerous.
Presently, hydrogen is stored using three strategies: high-pressure vaporous hydrogen stockpiling, low-temperature fluid hydrogen stockpiling, and strong state hydrogen capacity. Strong state materials are generally the most secure and provide the most hydrogen stockpiling thickness among materials with strong state hydrogen capacity.
“Following the initial burst effect, hydrogen migration and desorption are significantly easier. Structural engineering changes that enhance this desorption process could be the key to promoting MgH2 hydrogen desorption.”
Hao Li, associate professor at Tohoku University’s Advanced Institute for Materials Research (WPI-AIMR)
Metal hydrides have for quite some time been investigated for their enormous hydrogen stockpiling probability and their minimal expense. When these metals make contact with vaporous hydrogen, the hydrogen is consumed and deposited on the surface.Further energy input prompts hydrogen molecules to find their way into the metal’s precious stone grids until the metal becomes immersed in hydrogen. From that point, the material can assimilate and desorb hydrogen in larger amounts.
Magnesium hydride (MgH2) has shown massive commitment toward the predominant hydrogen stockpiling limit. Notwithstanding, a high temperature is fundamental for MgH2 to decay and deliver hydrogen. Moreover, the material’s perplexing hydrogen relocation and desorption, which bring about drowsy dehydrogenation energy, have frustrated its business application.
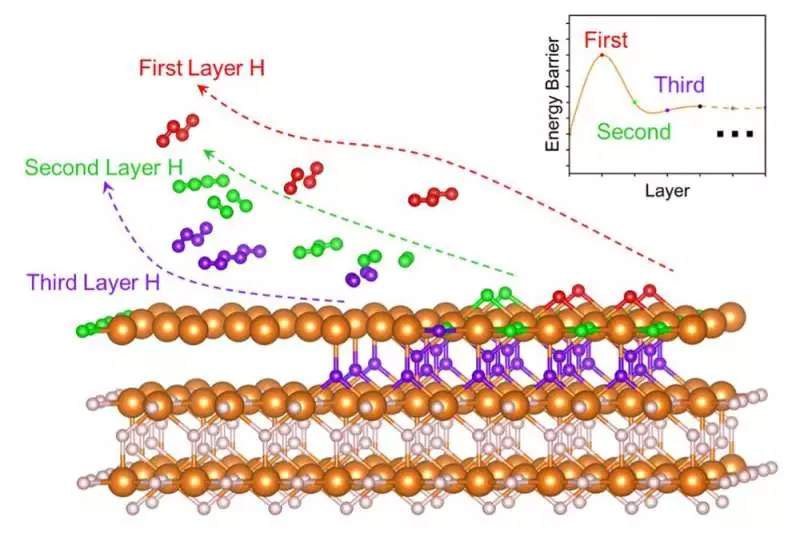
An intriguing “burst impact” on the dehydrogenation of MgH2, a normal strong state hydrogen capacity material, was discovered. After the slow dehydrogenation at the primary layer, hydrogen desorption from the resulting layers will be a lot more straightforward.
For quite a long time, researchers have discussed why dehydrogenation inside MgH2 is so troublesome. However, the investigation group has now revealed a response.
Utilizing estimations in view of the twist spellbound thickness utilitarian hypothesis with van der Waals remedies, they uncovered a “burst impact” during MgH2’s dehydrogenation. The underlying dehydrogenation hindrances were estimated at 2.52 and 2.53 eV, though the resulting response boundaries were 0.12-1.51 eV.
The group also conducted bond research using the precious stone orbital Hamilton population technique, and they discovered that the magnesium-hydride bond strength decreased as the dehydrogenation cycle progressed.
“Hydrogen relocation and hydrogen desorption are a lot more straightforward following the underlying burst impact,” brings up Hao Li, an academic administrator at Tohoku College’s High Level Establishment for Materials Exploration (WPI-AIMR) and the author of the paper. “Underlying changes that advance this desorption cycle could be the way to working with the hydrogen desorption of MgH2.”
Li and his colleagues demonstrated that when the main layer of nuclear hydrogen was present, hydrogen opportunities retained a significant level of electronic restriction.Investigations of the motor attributes of MgH2 after surface dehydrogenation, performed by stomach muscle initio sub-atomic element reproductions, likewise gave extra proof.
“Our discoveries give a hypothetical premise to MgH2’s dehydrogenation energy, giving significant rules to changing MgH2-based hydrogen-capacity materials,” adds Li.
More information: Shuai Dong et al, The “burst effect” of hydrogen desorption in MgH2 dehydrogenation, Journal of Materials Chemistry A (2022). DOI: 10.1039/D2TA06458H
Journal information: Journal of Materials Chemistry A





