Life on earth depends on repeating 24-hour natural cycles that are hereditarily encoded as atomic tickers in every single mammalian organ. Correspondence between these tickers has some control over circadian homeostasis. Fleeting coordination of digestion can then intervene between tissue correspondence. In another report currently distributed in Science Advances, Paul Petrus and a group of interdisciplinary scientists in epigenetics and digestion, wellbeing sciences, software engineering, and biomedicine at the University of California, Irvine, U.S., and Pompeu Fabra University in Barcelona, Spain, portrayed the cycle to which clocks across different organs control methodical metabolic rhythms. This direction is an examination region that until recently needed to be investigated. The group contemplated the metabolome of serum from mice with tissue-explicit articulation of the clock quality Bmal1. The trial results showed that the focal clock managed the metabolic rhythms by means of conduct. The discoveries featured the circadian association between tissues to stress the meaning of the focal clock overseeing the signs.
In a state of harmony with the pattern of the Earth.
The Earth twirls in a 24-hour cycle around its own pivot, and life has adjusted this transformative quality as a hereditarily encoded sub-atomic clock, alluded to as the center clock hardware. Each organ in the mammalian body has a clock that collaborates to direct circadian homeostasis. Interorgan correspondence depends on metabolic vacillations that think about the market interest of different tissues. The hidden premise of coordination for explicit tissue timekeepers to direct deliberate digestion remains a subject to be investigated. In this work, Petrus and the group investigated how the particular tissue timekeepers managed precise digestion by dissecting the jobs of neighborhood clocks to drive areas of strength for the metabolic soundness existing among serum, liver, and muscle in a cycle requiring as long as 24 hours to finish and coordinate to control circadian homeostasis.
Metabolic rhythms are connected with the center clock hardware containing a transcriptional-translational input circle that is synchronized for 24-hours. To examine this component, the group investigated time-explicit Bmal1 articulation in quality knockout creature models, where the qualities of premium were tentatively wrecked to comprehend the impact of explicit atomic systems on the center clock and on directing digestion.
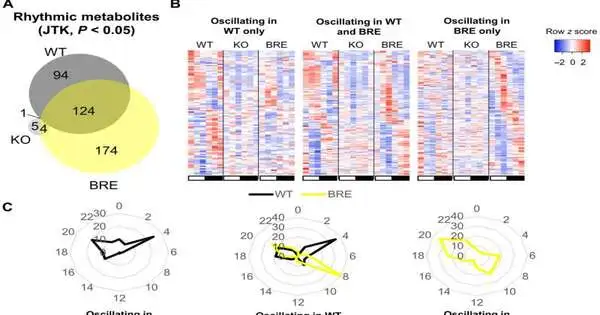
Most of the cadenced guidelines of coursing metabolites are driven by the focal clock. (A) Venn diagram depicting the cross-over of fundamentally important (JTK, P 0.05) serum metabolites in WT, Bmal1 KO, and Bmal1 cerebrum RE (BRE) mice.(B) Heatmaps addressing the swaying metabolites in WT alone (left heatmap), in WT and BRE together (center heatmap), and in BRE alone (right heatmap).(C) Radar plots showing the duration of metabolic motions in WT alone (left radar plot), between WT and BRE (center radar plot), and in BRE alone (right radar plot).(D) A graph depicting the amplitudes of the covering metabolites in WT and BRE.distinction was surveyed by a matched t test. (E) The adjustment of sufficiency within each pathway in BRE mice compared to WT.(F) Pathway enhancement of swaying metabolites covers both WT and BRE. (G) phase of metabolites in the major swaying metabolic classes. *P 0.05.All investigations were performed utilizing n = 4 mice.
The experiments
During the investigations, the scientists euthanized mice like clockwork inside a 24-hour diurnal cycle and gathered serum from all of the mice’s partners. They investigated the serum utilizing worldwide metabolomics through fluid chromatography mass spectrometry (LC/MS). While the wild kind, otherwise called “ordinary” mice partners, showed huge circadian motions recognized through flowing metabolites, the Bmal1 knockout mice associates lost rhythmicity of all metabolites seen in the wild sort, with the exception of cysteine-S-sulfate. The outcomes affirmed the significance of Bimal1 articulation for motions in circulatory metabolites. The review results reaffirmed how nearby fringe timekeepers in disconnection are lacking to drive most of the circadian metabolic results into course, further highlighting the reliance of a greater part of circulatory metabolites on other tissue tickers or the correspondence between tissue clocks.
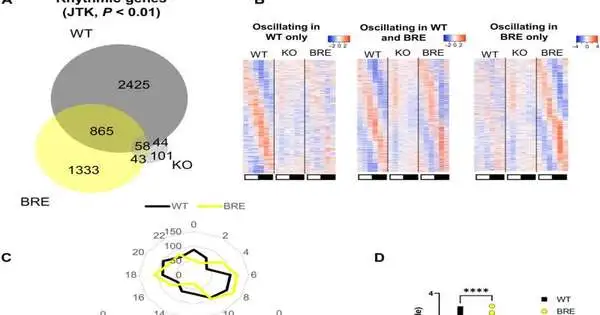
The focal clock is adequate to drive circadian rhythms of the hepatic record and to reestablish glucose resistance. (A) Venn chart addressing the cross-over of fundamentally swaying (JTK, P 0.01) liver records between WT, Bmal1 KO mice, and Bmal1 BRE. (B) Heatmaps addressing the wavering records in WT only (left heatmap), WT and BRE combined (center heatmap), and BRE only (right heatmap).(C) Radar plots delineating the period of transcriptional motions in WT alone (bottom left radar plot), between WT and BRE (top radar plot), and in BRE alone (bottom right radar plot).(D) Graph displaying the amplitudes of the covering wavering records between WT and BRE.distinction was evaluated by a matched t test. (E) Improvement of swaying record covers pathways in WT and BRE mice.(F) oral glucose tolerance test (OGTT) and determined region under the curve (AUC) in all genotypes discussed in the review.Tremendous contrasts were investigated by a one-way examination of change (ANOVA) followed by Fisher’s most un-huge distinction post hoc test. P 0.05, **P 0.01, ***P 0.001, and ***P 0.0001. All examinations were performed utilizing mainly n = 3 mice.
Brain clocks
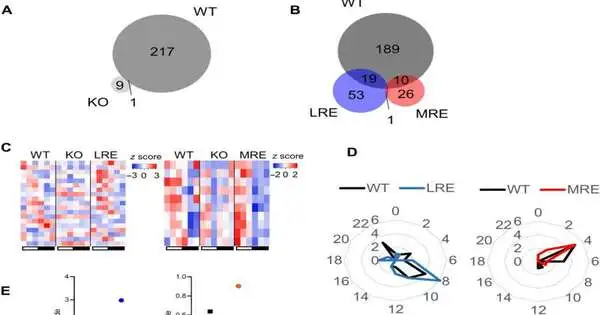
Metabolic rhythms, as a rule, flourish with food consumption or from stores of energy inside the body. Cadenced behavior is thus a determinant of fundamental metabolic motions.Conduct rhythms are typically managed by the focal clock of the suprachiasmatic core, inside which the focal circadian pacemaker is found and profoundly communicates the Syt10 quality. Taking out Bmal1 in Syt10 communicating neurons brought about arrhythmic conduct in mice set in steady haziness. Following additional tests, the group reestablished Bmal1 articulation in Syt10 neurons to determine whether the cycle reestablished social rhythms comparable to locomotor action, digestion, and taking care of behavior when contrasted with wild type and knockout creature models.The researchers saw to some degree safeguarded body weight for the mice, including all out action, food admission, and adiposity as patterns toward salvage. The circadian rhythms compared with conduct and digestion were additionally, to some extent, reestablished. The fractional reclamation stressed the prerequisite for tickers in other cell kinds of the mind for the full rebuilding of social rhythms. The information likewise proposed the impact of the focal clock to drive most circadian coursing metabolic rhythms, while the stage and adequacy required extra guidelines through different timekeepers.
Digestion and the center clock
The specialists further concentrated on how much the focal clock could control transcriptional motions without fringe tickers, by means of RNA-sequencing reads for over 24-hours. A few components gave off an impression of being managed through fundamental digestion, free from the center clock hardware. They further showed how glucose digestion depends on the circadian framework, while foundational glucose homeostasis controls clocks in various organs. The outcomes underscored the meaning of controlling glucose homeostasis and, surprisingly, gave proof of how shift work is connected to diabetes. The group additionally concentrated on the cycle by which the focal clock controlled foundational digestion. The findings demonstrated that taking care of musicality could protect the circling metabolic mood of more than 56% Bmal1-kcnockout mice.
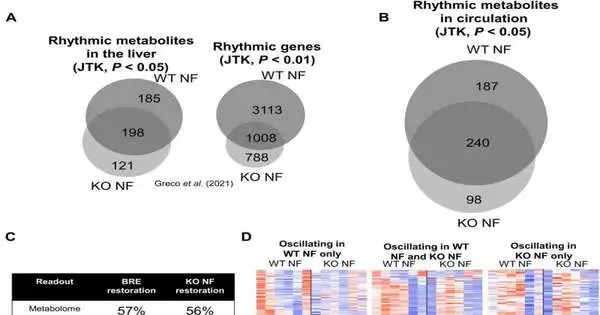
Serum metabolite motions are predominantly determined by taking care of rhythms. (A) Venn diagram addressing the cross-over of essentially swaying liver metabolites (JTK, P 0.05) and records (JTK, P 0.01) among WT and Bmal1 KO mice exposed to NF. These are specially appointed examinations by openly accessible datasets from our past papers. (B) Venn diagram illustrating the cross-over of significantly influencing serum metabolites (JTK, P 0.05) in WT and Bmal1 KO mice exposed to NF. (C) Comparison of the extent of metabolites and records reconstituted after once again introducing the clock in the SCN or NF (D) Heatmaps addressing the swaying records in WT NF only (left heatmap), WT NF and KO NF combined (center heatmap), and KO NF only (right heatmap). (E) Radar plots of the period of circulatory metabolic motions in WT NF alone (left radar plot), in WT NF and KO NF combined (center radar plot), and in KO NF alone (right radar plot). (F) Graph comparing the amplitudes of covering swaying metabolites in WT and KO NF. Massive distinction was evaluated by a matched t test. (G) phase of metabolites in the major wavering metabolic classes. ***P 0.001.All examinations were performed utilizing n = 4 mice.
Outlook
Along these lines, Paul Petrus and associates analyzed the intricate components of circadian organ correspondence. They showed how the focal clock drove fundamental rhythms generally by directing the taking care of fasting rhythms. The work stressed food as a significant synchronizing factor, adding to the job of the suprachiasmatic core as an expert pacemaker. The work presents the focal center clock as a driver of fundamental metabolic rhythms. Future work will reveal insight into deciphering fringe tickers to carry out clinical treatments to treat disturbed circadian rhythms.
More information: Paul Petrus et al, The central clock suffices to drive the majority of circulatory metabolic rhythms, Science Advances (2022). DOI: 10.1126/sciadv.abo2896
Kenneth A. Dyar et al, Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks, Cell (2018). DOI: 10.1016/j.cell.2018.08.042
Journal information: Science Advances