In a record-breaking experiment, a next-generation flow battery design’s capacity and longevity were demonstrated to be enhanced by a common food and medicine additive.
An exploration group from the Branch of Energy’s Pacific Northwest Public Research Facility reports that the stream battery, a plan upgraded for electrical network energy capacity, kept up with its ability to store and deliver energy over an extended period of persistent charge and release.
The study, which was recently published in the journal Joule, describes the first application of a starch-derived dissolved simple sugar called cyclodextrin to increase battery capacity and longevity. The scientists tweaked the system’s chemical composition through a series of tests until it produced 60% more peak power.
“We demonstrated that a completely different kind of catalyst may be used to quicken the energy conversion. Furthermore, since it is dissolved in the liquid electrolyte, there is little chance that a solid may dislodge and clog the system.”
Wei Wang, a long-time PNNL battery researcher and the principal investigator of the study.
The experiment was then put on hold for more than a year when the plastic tubing failed, after which they repeatedly cycled the battery. The flow battery barely lost any of its capacity to recharge during all of that time. This is the first laboratory-scale flow battery experiment to report minimal capacity loss after more than a year of continuous use.
In a process known as homogeneous catalysis, the cyclodextrin additive is also the first to accelerate the electrochemical reaction that stores and then releases the flow of battery energy. This indicates that the sugar performs its function when dissolved in solution as opposed to when applied as a solid to a surface.
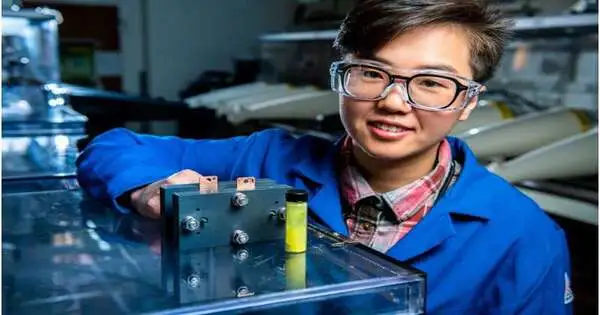
Scientists set up an exploratory stream battery electrolyte that has shown long life in the research center setting. Credit: Sara Levine, Pacific Northwest Public Lab.
“This is a pristine way to deal with creating stream battery electrolyte,” said Wei Wang, a long-term PNNL battery specialist and the central examiner of the review. “We demonstrated that a completely different kind of catalyst designed to speed up energy conversion can be used. What’s more, further, in light of the fact that it is disintegrated in the fluid electrolyte, it takes out the chance of a strong dislodging and fouling the framework.”
A flow battery: what is it?
Flow batteries have two chambers, each filled with a different liquid, as their name suggests. The batteries store energy in chemical bonds and charge through an electrochemical reaction. They release that energy, which can power electrical devices, when they are connected to an external circuit. In contrast to solid-state batteries, flow batteries have two external supply tanks that constantly circulate liquid to supply the electrolyte—similar to the system’s “blood supply.” The bigger the electrolyte supply tank, the more energy the stream battery can store.
Flow batteries can be used as backup generators for the electric grid if they are expanded to the size of a football field or more. Stream batteries are one of the critical mainstays of a decarbonization technique to store energy from sustainable power assets. They are advantageous because they can be constructed at any scale, from a laboratory bench, as in the PNNL study, to a city block.
For what reason do we want new sorts of stream batteries?
A kind of insurance policy against disruptions to our electrical grid is provided by large-scale energy storage. At the point when serious climate or popularity limit the capacity to supply power to homes and organizations, energy put away in enormous scope stream battery offices can assist with limiting disturbance or reestablishing administration. As the use of renewable energy sources like wind, solar, and hydroelectric power increases, the demand for flow battery facilities is only expected to rise. These kinds of intermittent power sources necessitate a location where energy can be stored until it is required to meet consumer demand.
Even though there are a lot of different flow battery designs and some commercial installations, the minerals like vanadium that are mined and used in commercial facilities are expensive and hard to get. Because of this, teams of researchers are looking for viable alternatives that make use of more common materials that are simple to synthesize, stable, and non-toxic.
Stream battery scientist Ruozhu Feng presents elements for an enduring lattice energy battery. Credit: Andrea Starr | Pacific Northwest National Laboratory
“We cannot always dig the Earth for new materials.” Andrea Starr, Pacific Northwest National Laboratory We really want to foster a reasonable methodology with synthetics that we can orchestrate in huge sums—very much like the drug and food businesses.” said Imre Gyuk, director of energy storage research at DOE’s Office of Electricity.
The work on stream batteries is essential for an enormous program at PNNL to create and test new innovations for network-scale energy capacity that will be advanced rapidly with the kickoff of PNNL’s Matrix Stockpiling Platform in 2024.
An effective flow battery is sweetened by benign “sugar water.” Members of the PNNL research team with backgrounds in organic and chemical synthesis developed this new battery design. When the team decided to work with materials that were already produced for other industrial purposes but had not been used in battery research, these skills came in handy.
“We were searching for a straightforward method for dissolving more fluorenol in our water-based electrolyte,” said Ruozhu Feng, the main creator of the new review. “The -cyclodextrin did that humbly, but its genuine advantage was this astounding synergistic capacity.”
After that, to explain how the catalytic boost works, the researchers collaborated with co-author Sharon Hammes-Schiffer of Yale University, a leading authority on the chemical reaction behind it.
The sugar additive accepts positively charged protons, which helps to balance out the movement of negative electrons as the battery discharges, as the research study explains. Although the specifics are a little more nuanced, it is comparable to when sugar sweetens the pot to permit the other chemicals to complete their chemical dance.
The review is about the up-and-coming age of a PNNL-protected stream battery configuration previously portrayed in the diary Science in 2021. There, the researchers demonstrated that fluorenone, a common chemical, is an efficient flow battery component. However, the initial breakthrough needed to be improved due to the process’s slowness in comparison to flow battery technology that was already in use. According to the researchers, this new development makes the battery design suitable for scaling up.
Simultaneously, the exploration group is attempting to additionally work on the framework by exploring different avenues regarding different mixtures that are like -cyclodextrin yet more modest. Like honey, -cyclodextrin expansion additionally makes the fluid thicker, which is not so great for a streaming framework. In any case, the specialists found that its advantages offset its downsides.
Numerous scientists, including Ying Chen, Xin Zhang, Peiyuan Gao, Ping Chen, Sebastian Mergelsberg, Lirong Zhong, Aaron Hollas, Yangang Lian, Vijayakumar Murugesan, Qian Huang, Eric Walter, and Yuyan Shao of PNNL, and Benjamin J. G. Rousseau and Hammes-Schiffer of Yale, in addition to Feng and Wang, were needed to comprehend the intricate
The exploration group has applied for U.S. patent assurance for their new battery plan.
More information: Ruozhu Feng et al, Proton-regulated alcohol oxidation for high-capacity ketone-based flow battery anolyte, Joule (2023). DOI: 10.1016/j.joule.2023.06.013





