The majority of liquids either solidify or crystallize when cooled to their freezing point. To put it another way, the molecules arrange themselves perfectly, creating what physicists refer to as a crystal. Liquids at supercooling temperatures differ. Even when they are cooled below their freezing point, they do not form crystals of this kind. Although these liquids are utilized in numerous industries, their properties are poorly understood. The researchers at Eindhoven University of Technology (TU/e) have used four body correlation functions for the first time to present the most accurate description of their properties to date. The work is distributed in the PNAS Nexus diary.
Examine the surroundings. What do you see? Are there magazines, newspapers, or books around? Or, when you’re outside, you can see as far as the eye can see: fields, plants, and trees. When everything is neatly arranged, it can be pleasing to the eye. However, when things become muddled or disorganized, it can be unpleasant and messy for many people.
It is simple to determine whether a system is organized or messy in the macroworld. However, there is also the difficulty of zooming in on the scale of atoms and molecules in substances. Liquids, on the other hand, do not typically have an ordered crystalline structure.
“A supercooled liquid is one that can be cooled or compressed below its freezing point without solidifying.”
Ilian Pihlajamaa, Ph.D., researcher in the Soft Matter & Biological Physics group at TU/e.
Normal liquids versus supercooled liquids
Compared to supercooled liquids, normal liquids have molecules that are constantly moving and are free to explore the volume that is enclosing them. A normal liquid’s temperature is above its freezing point but below its boiling point. To put it another way, the positions of the molecules in relation to one another are not clearly defined.
However, a phase transition occurs when the liquid molecules arrange themselves in a very specific manner to form a crystalline solid once it has been cooled to its freezing point.
When the temperature is below zero degrees Celsius, water’s molecules are locked in a crystalline solid phase (ice), but when the temperature is above zero degrees Celsius, they can move freely. Supercooled liquids, on the other hand, present a completely different challenge.
According to TU/e researcher Ilian Pihlajamaa, Ph.D., “a supercooled liquid is one that can be cooled down or compressed below its freezing point without turning into a crystal.”
Why, then, does this occur? “In some cases, if the cooling is very fast, the liquid can remain in a liquid state even if the temperature is below the normal freezing point,” says Corentin Laudicina, Ph.D., a colleague of Pihlajamaa at TU/e. As a result, it has some unique properties that can be useful in a variety of industrial applications, including high-tech optical materials and sustainable plastics.”
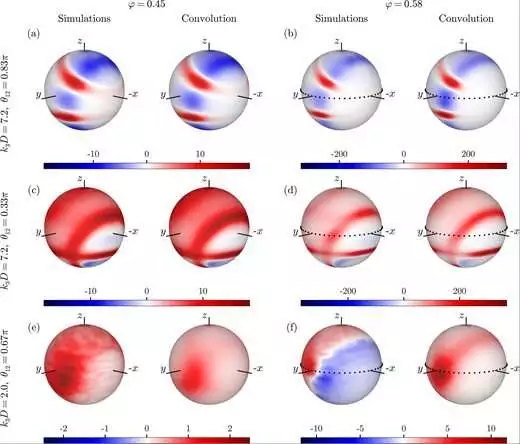
Four-point structure factor convolution approximations and simulation results for normal (left) and supercooled (right) liquids are compared. Credit: PNAS Nexus (2023). DOI: 10.1093/pnasnexus/pgad184
Going beyond pairs
Because supercooled liquids are used in so many different ways, it would be helpful to know more about how the molecules in supercooled liquids interact with one another. These interactions can have a significant impact on the properties of the liquid as a whole.
One of the most well-known instruments to depict a fluid’s design is a two-particle or two-body underlying connection, which looks at how any two atoms influence or correspond with one another. As would be normal by instinct, particles that are nearer will generally have a more noteworthy impact on a particle of interest than atoms that are further away from the atom of interest.
“We would like to know more about the influence of multiple molecules on a given molecule at the same time,” Pihlajamaa states. “Two-molecule correlations are useful.” Multiple-molecule correlations are required to fundamentally describe a liquid’s structure in greater depth. Moving past two-and three-atom relationships gives these subtleties.”
Pihlajamaa and Laudicina, along with their supervisors Liesbeth Janssen from TU/e and Chengjie Luo from the Max Planck Institute for Dynamics and Self-Organization in Göttingen, calculated many-body correlations using simulations and a new theory in the paper that was published in PNAS Nexus.
Problems with calculation:
“The majority of studies to this point only focus on structural correlations between two bodies, and only a few papers have attempted to consider additional correlations between three bodies.” “Being the first to look at four-body correlations, we have gone further than anyone else in terms of the calculations,” states Janssen.
We are aware that supercooled liquids possess distinct characteristics from normal liquids. According to Laudicina, “the laws of physics indicate that these properties are connected to the liquid’s molecular structure.” For supercooled liquids, the two- and three-molecule correlations are similar, but the four-molecule correlations reveal previously unknown locally preferred structures.
The research presented the researchers with a number of difficulties. The numerical inferences were genuinely lengthy and specialized,” says Laudicina. ” “Verifying whether or not this was correct presented an interesting challenge because we had to deal with tens to hundreds of terms in the equations,” adds Pihlajamaa.
Added to that, to quantify how a four-body connection works, the scientists required loads of information. “Our in-house-developed code was executed by sophisticated computer programs running on high-performance clusters. Our PCs were most certainly not equipped for running this code,” says Pihlajamaa.
Twenty years and counting
This was a significant breakthrough for the researchers, especially considering that the four of them have worked on better theories to explain the correlations between molecules in liquids for more than two decades.
According to Janssen, “breakthroughs such as this will inspire me to continue working on these novel ideas further and to improve upon the best theories that are out there.”
Laudicina adds, “Bringing a sense of fulfillment and satisfaction is contributing to the understanding of the complex behavior of dense liquids and pushing the boundaries of what is currently known.”
New materials could be developed for use in materials engineering, manufacturing processes, chemical engineering, and the design of energy storage systems if a deeper understanding of the structure of supercooled liquids is gained.
“It’s all about making new materials work better and last longer.” However, in order to accomplish this, it is essential to enhance our comprehension of the fundamental aspects of these systems, and our research is a significant step in the right direction,” explains Janssen.
More information: Ilian Pihlajamaa et al, Emergent structural correlations in dense liquids, PNAS Nexus (2023). DOI: 10.1093/pnasnexus/pgad184