An internal heat level is an essential sign of wellbeing. Intracellular temperature is likewise an essential sign of cell well-being; malignant growth cells are all the more metabolically dynamic, and in this way, can have a somewhat higher temperature than sound cells. However, as of recently, the accessible instruments for testing such speculations haven’t been capable. In a recent article published in Nano Letters, researchers from Osaka University and their collaborators tentatively estimated temperature slopes inside human cells with remarkable accuracy.This study will lead to new headings in drug revelation and clinical examination.
Numerous scientists have thought that transient intracellular temperature inclinations more broadly affect human wellbeing than normally appreciated, yet they couldn’t test their speculations inferable from the limits of the innovation accessible to them. “Ebb and flow intracellular warm discovery innovation has inadequate spatial, transient, and readout goals to answer a few well established clinical speculations,” makes sense of Kai Lu, lead writer, “yet our exploration changes this.” Our hereditarily encoded fluorescent nanothermometer conquers earlier specialized obstacles and will be important for testing such speculations. “
“Our research changes this because the current intracellular thermal detection technology lacks the spatial, temporal, and readout resolution to address some long-standing medical hypotheses. Our genetically encoded fluorescent nanothermometer, which will be invaluable for testing such hypotheses, overcomes earlier technical challenges.”
Kai Lu, lead author,
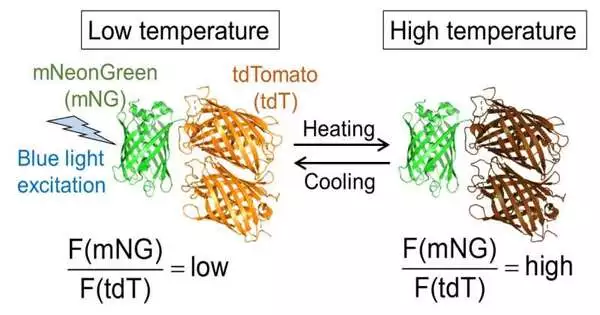
The scientists’ protein-put together nanothermometer is based on adjusted fluorescence yield that is delicate to little changes in temperature inside cells. Its readout speed is multiple times faster than similar innovations and multiple times faster than a standard squint of your eye.The nanothermometer enabled the scientists to find that intracellular intensity dispersion is in excess of multiple times slower than heat dissemination in water. It likewise showed that the readout goal is just 0.042 degrees Celsius at physiological temperature, which is a considerably higher goal than that in a practically identical arrangement that is a few thousand times slower.
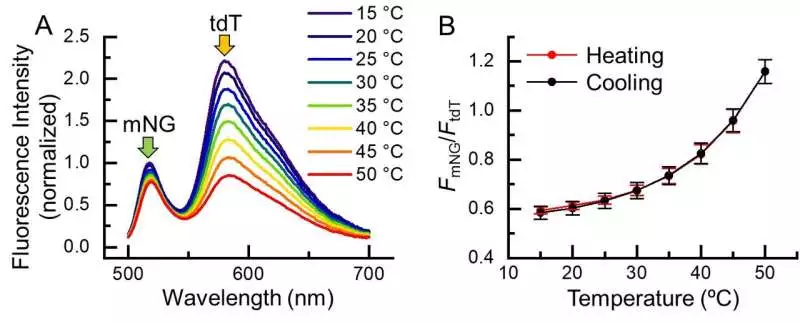
Fig. 2. Temperature reaction of B-gTEMP. (A) Temperature-dependent fluorescence range of B-gTEMPtdT: tdTomato; mNG: mNeonGreen.(B) Fluorescence power proportion of mNG to tdT in relation to temperature during a warming and cooling pattern.
“We tried the speculation that there’s a significant temperature contrast between the cell core and cytoplasm,” says Takeharu Nagai, senior creator. “We didn’t track down a huge distinction, however, test conditions that all the more intently copy run-of-the-mill physiology could give various outcomes.”
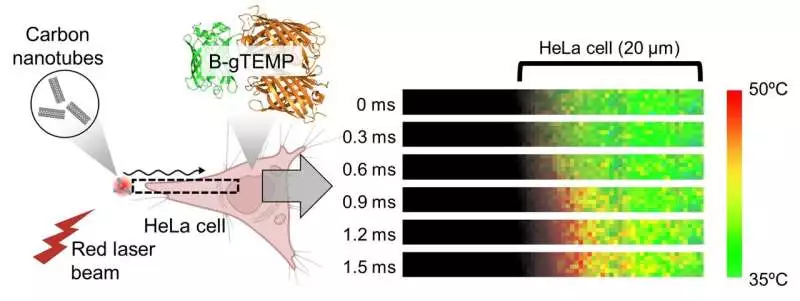
Fig. 3. Quick-intensity transport in cells. Heat was produced by lighting carbon nanotubes with an engaged red laser pillar; the intensity then diffused into the contiguous HeLa cell. This cycle was caught progressively by kilohertz temperature imaging with B-gTEMP.
There are a few methods for working on the usefulness of the scientists’ nanothermometer. One is to further develop how long it endures under tiny brightening. Another is to reengineer it to be delicate to red or infrared light, and consequently be less harmful to cells for long-haul imaging. Meanwhile, scientists currently have the innovation to practically test intracellular temperature angles and reveal the physiology that supports these slopes. Maybe with this information, medications could one day at any point be intended to exploit this undervalued part of cell physiology.
More information: Kai Lu et al, Intracellular Heat Transfer and Thermal Property Revealed by Kilohertz Temperature Imaging with a Genetically Encoded Nanothermometer, Nano Letters (2022). DOI: 10.1021/acs.nanolett.2c00608
Journal information: Nano Letters