Heterocyclic mixtures are natural particles with a ring structure containing something like at least two components. Generally speaking, these rings are made out of carbon molecules alongside at least one different component like nitrogen, oxygen, or sulfur. They are exceptionally pursued as natural substances in the compound and drug industry, attributable to their flexibility and phenomenal physiological exercises.
While a few techniques are accessible for integrating these mixtures, the majority of them include high temperature and tension circumstances or the utilization of valuable metal impetuses, adding to the monetary and natural expense of creating heterocyclic natural mixtures.
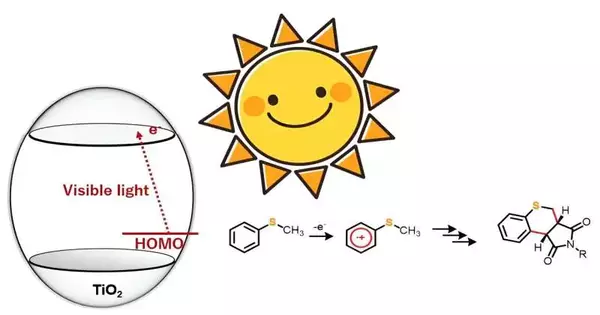
Presently, in any case, a group of scientists from Japan and Bangladesh have proposed a straightforward yet powerful technique for defeating these difficulties. Their review was, as of late, distributed in the diary Progressed Amalgamation and Catalysis. Utilizing the proposed technique, the group showed the amalgamation of 20 sulfur-containing heterocyclic mixtures within the sight of photocatalyst titanium dioxide (TiO2) and noticeable light.
“We discovered that, while ultraviolet light generates highly oxidative holes, our method allows for the selective one-electron oxidation of substrate molecules using visible light. This technique can thus be used in a variety of organic chemical processes.”
Professor Yutaka Hitomi from the Department of Applied Chemistry, Graduate School of Science and Engineering, Doshisha University,
The review was driven by Teacher Yutaka Hitomi from the Division of Applied Science, Graduate School of Science and Designing, Doshisha College, and co-created by Ph.D. up-and-comer Pijush Kanti Roy from Doshisha College, Academic Administrator Sayuri Okunaka from Tokyo City College, and Dr. Hiromasa Tokudome from Exploration Foundation, TOTO Ltd.
TiO2 as a photocatalyst for driving natural responses has caught the attention of manufactured scientific experts for some time now. In any case, many such cycles require bright light to set off the response. In this review, notwithstanding, the examination group found that under anaerobic circumstances, sulfur-containing natural mixtures like thioanisole subordinates, when hit with blue light, responded with maleimide subsidiaries to frame double carbon securities, yielding another heterocyclic natural compound.
“We saw that while bright light creates exceptionally oxidative openings, our methodology takes into consideration the particular one-electron oxidation of the substrate atoms utilizing apparent light. This approach can accordingly be utilized in different natural synthetic responses,” makes sense to Prof. Hitomi.
The analysts picked five 4-subbed thioanisoles and four N-subbed maleimides for the annulation or ring development responses. The group illuminated the beginning material with blue light (frequency > 420 nm) but noticed no response. Nonetheless, bringing TiO2 into the response framework prompted the union of 20 unique thiochromenopyrroledione subsidiaries with a moderate-to-high return. They tracked down that in something like 12 hours of openness to blue light, the response among thioanisole and N-benzylmaleimide prompted the development of a thiochromenopyrroledione subsidiary with a 43% yield, which was near the hypothetical most extreme yield of half.
The examination group likewise noticed substituent impact in the responses to figure out the relating robotic angles. From the outcomes, they hypothesized that the response continues through a charge move from thioanisole to the conduction band of TiO2. Moreover, they proposed that illumination with blue light set off one-electron oxidation of thioanisole, which further started the age of α-thioalkyl revolutionaries through deprotonation.
In outline, this new and refined approach exhibits the capability of TiO2 for noticeable light photocatalysis for natural blends. It additionally gave urgent insights into the science of perplexing heterocyclic compound combinations. Pushing ahead, this approach can open up additional opportunities for changing from the current serious modern synthetic cycles to a more energy-productive framework.
Prof. Hitomi says, “What drove our review was the craving to help with the improvement of a manageable compound industry, and our discoveries have all the earmarks of being a positive move toward this bearing.”
“We accept that the far and wide reception of this apparent light-determined innovation could aid an available and reasonable blend of drugs, with its significant effects on the wellbeing and prosperity of millions of individuals around the world.” On account of the endeavors of Prof. Hitomi and his group, their review has opened up new roads in the field of natural union, with the possibility of altering different substance enterprises.
More information: Pijush Kanti Roy et al. Blue Light-Promoted Synthesis of Thiochromenopyrroledione Derivatives via Titanium Dioxide‐Catalyzed Dual Carbon-Carbon Bond Formation with Thioanisole and Maleimide Derivatives, Advanced Synthesis & Catalysis (2023). DOI: 10.1002/adsc.202301021