Converting solar energy into hydrogen energy is a promising and environmentally friendly method of addressing the energy crisis and reducing petroleum product outflows.An exploration group from City College of Hong Kong (CityU) has of late fostered a sans-lead perovskite photocatalyst that conveys profoundly effective sun-based energy-to-hydrogen change.
In particular, they divulged the interfacial elements of strong (between halide perovskite particles) and strong fluid (between a halide perovskite and an electrolyte) interfaces during photoelectrochemical hydrogen creation. The most recent discoveries open up a road to foster a more effective, sun-driven strategy for creating hydrogen fuel from now on.
Because of its abundance, high energy density, and natural cordiality, hydrogen is regarded as a superior and truly encouraging sustainable power option.Aside from photoelectrochemical water splitting, another promising method for producing hydrogen is by splitting hydrohalic corrosive with sun-powered photocatalysts.Yet, the drawn-out strength of photocatalysts is a basic test, as most catalysts made of metal oxides or metal are shaky under acidic circumstances.
“While lead-based hybrid perovskites are employed to circumvent this stability issue, their high water solubility and toxicity limit their promise for widespread use.”
Dr. Sam Hsu Hsien-Yi, Assistant Professor in the School of Energy and Environment
“Toxic mixture perovskites are used to overcome this security issue, but their high water solubility and lead toxicity limit their true capacity for broad applications,” explained Dr. Sam Hsu Hsien-Yi, an assistant professor in CityU’s School of Energy and Climate and the Branch of Materials Science and Designing.
“Bismuth-based perovskites, on the other hand, have been confirmed to provide a non-harmful, synthetically stable option for solar fuel applications, though photocatalytic proficiency should be improved.”
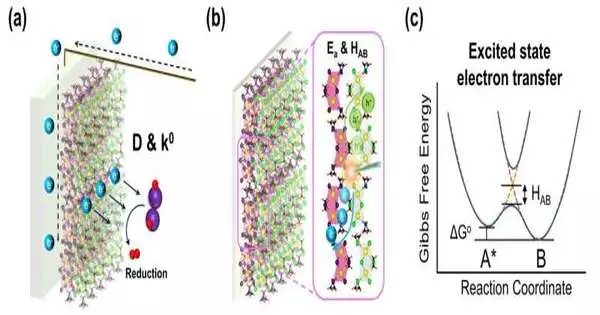
Dr. Hsu and his colleagues recently developed a bismuth-based halide perovskite with a bandgap piping design for profoundly effective charge-transporter transport in their quest for an effective and stable photocatalyst.It is a blended halide perovskite, in which the conveyance of iodide particles slowly diminishes from the surface to the inside, shaping a bandgap pipe structure that advances a photograph-prompted charge move from the inside to the surface for an effective photocatalytic redox response.
This recently planned perovskite has high sun-based energy change proficiency, showing a hydrogen age rate improvement up to around 341 61.7 mol h1 with a platinum co-impetus under noticeable light illumination. The discoveries were distributed in “Little Strategies.”
Yet, Dr. Hsu’s group didn’t stop there. “We needed to investigate the unique connections between the halide perovskite particles and those at the point of interaction between the photoelectrode and the electrolyte, which remained obscure,” said Dr. Hsu.
“Since photoelectrochemical hydrogen creation includes a reactant cycle, profoundly viable hydrogen aging can be accomplished by serious light ingestion involving a semiconductor as a photocatalyst with a reasonable energy band structure and effective charge division, worked with by an outer electrical field framed close to the semiconductor-fluid connection point.”
The group used temperature-subordinate time-settled photoluminescence to dissect the energy transportation of electron-opening matches between perovskite atoms to find the exciton-move elements.They also examined the dispersion coefficient and electron move rate constant of halide perovskite materials in the solution to demonstrate the viability of electron transport through the strong fluid connection points between a perovskite-based photoelectrode and the electrolyte.
“We demonstrated how our recently planned photocatalyst can truly achieve elite execution photoelectrochemical hydrogen age due to an effective charge move,” Dr. Hsu said.
In the trial, the group likewise demonstrated that bandgap piping organized halide perovskites for a more effective charge division and transfer process between the connection point of the cathode and electrolyte.
The better charge division can drive the movement of charge transporters onto the outer layer of halide perovskites kept on the conductive glasses as the photoelectrode, permitting quicker photoelectrochemical action on the photoelectrode’s surface. Thus, the viable charge moved inside the bandgap pipe, and organized halide perovskites showed improved photocurrent thickness under light illumination.
“Revealing the interfacial elements of these clever materials during the photoelectrochemical hydrogen age is a critical forward leap,” Dr. Hsu reasoned.”A thorough understanding of the interfacial connections between halide perovskites and fluid electrolytes can serve as a logical starting point for scientists in this field to further investigate the development of options and useful materials for solar-powered hydrogen generation.”
The discoveries were distributed in cutting-edge materials.
More information: Yunqi Tang et al, Unravelling the Interfacial Dynamics of Bandgap Funneling in Bismuth‐Based Halide Perovskites, Advanced Materials (2022). DOI: 10.1002/adma.202207835
Yunqi Tang et al, Bandgap Funneling in Bismuth‐Based Hybrid Perovskite Photocatalyst with Efficient Visible‐Light‐Driven Hydrogen Evolution, Small Methods (2022). DOI: 10.1002/smtd.202200326