High-impact glycolysis, the cycle by which cells change glucose into lactate, is key for eye improvement in well-evolved creatures, as per another Northwestern Medication Concentrate distributed in Nature Correspondences.
While it has been noted that retinal cells use lactate during cell separation, the specific role that this cycle plays in early eye improvement was not recently perceived.
Guillermo Oliver, Ph.D., senior author of the study and the Thomas D. Spies Professor of Lymphatic Metabolism and Director of the Feinberg Cardiovascular and Renal Research Institute Center for Vascular and Developmental Biology, claims that the findings contribute to a better understanding of the metabolic pathways that underlie organ development.
“For quite a while, my lab has been keen on formative science. Particularly, Oliver stated, “to characterize the molecular and cellular steps that regulate early eye morphogenesis.” As far as we might be concerned, the inquiry was: ‘ How do these remarkable and essential facial sensory organs begin to develop?
“We discovered that the glycolytic pathway has an ATP-independent role. Lactate, hitherto known as a waste product, is now performing some interesting things in ocular morphogenesis. This clearly indicates that this molecule plays an important role in organ morphogenesis, particularly eye morphogenesis. This discovery has larger significance, as it is anticipated to be necessary in other organs, as well as regeneration and illness.”
Nozomu Takata, Ph.D., a postdoctoral fellow in the Oliver lab.
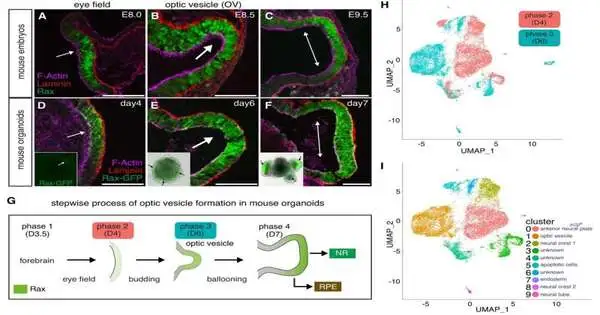
Nozomu Takata, Ph.D., a postdoctoral individual in the Oliver lab and the first creator of the paper, at first moved toward this inquiry by creating undeveloped, immature microorganism-determined eye organoids, which are organ-like tissues designed in a petri dish. Intriguingly, he saw that early mouse eye begetters showed raised glycolytic action and the creation of lactate.
In the wake of acquainting a glycolysis inhibitor with the refined organoids, typical optic vesicle improvement ended, as per the review, yet adding back lactate permitted the organoids to continue typical eye morphogenesis, or improvement.
Takata and his colleagues then contrasted those organoids with controls utilizing a vast transcriptome and epigenetic investigation utilizing RNA and ChIP sequencing. They discovered that adding lactate to the organoids and inhibiting glycolysis controlled the expression of some essential, evolutionary-conserved genes for early eye development.
To approve these discoveries, Takata erased Glut1 and Ldha, qualities known for managing glucose transport and lactate creation, from creating retinas in undeveloped mouse organisms. According to the study, the deletion of these genes halted normal glucose transport in the eye-forming region specifically.
“What we found was an ATP-free job for the glycolytic pathway,” Takata said. “Lactate, which is a metabolite known as a byproduct previously, is truly accomplishing something cool in eye morphogenesis. This clearly demonstrates that this metabolite plays a significant role in organ morphogenesis, specifically eye morphogenesis. I view this disclosure as having more extensive ramifications, as probable additionally being expected in different organs and perhaps in recovery and sickness also.”
Following this revelation, Takata said he intends to keep on exploiting conventional and arising formative science’s apparatuses, for example, mouse hereditary qualities and undifferentiated cells determined organoids, to concentrate on the job of the glycolytic pathway and digestion in the improvement of different organs.
Oliver stated that the findings may also be useful in gaining a deeper understanding of the direct effect that metabolites may have on regulating gene expression during tumor development and organ regeneration.
“Both recovery and tumorigenesis include formative pathways that turn out badly in certain events or that you really want to reactivate,” Oliver said. “For the majority of formative cycles, you really want extremely severe transcriptional guidelines.” A quality is on or off at specific times, and when that turns out badly, that could prompt formative imperfections or advance tumorigenesis. The fact that we now know which specific metabolites are responsible for normal or abnormal gene regulation can help us think more broadly about treatment options.”
More information: Nozomu Takata et al, Lactate-dependent transcriptional regulation controls mammalian eye morphogenesis, Nature Communications (2023). DOI: 10.1038/s41467-023-39672-2