Analysts from the Weizmann Institute of Science and the U.S. Branch of Energy’s (DOE) Brookhaven National Laboratory have researched the sub-atomic level elements at play when methanol converts to hydrogen by means of the assistance of a copper impetus, with the entire framework under surrounding tension and temperature. This review, which yielded some amazing data about the way of behaving of the methanol on various copper surfaces, will assist specialists with finding the best-performing copper impetus for this interaction and, all the more comprehensively, further understanding how they might collectively interpret copper impetuses.
Methanol (a compound of hydrogen, carbon, and oxygen) is a flexible material in the energy business. It may very well be utilized as a fuel itself or, as in this exploration, to deliver another fuel: hydrogen. Methanol is the main possibility for hydrogen capacity advances, especially in car energy component applications. A fluid at room temperature, it is not difficult to work with and viable with the current gas framework. It likewise holds a somewhat enormous measure of hydrogen by volume. Yet, to proficiently deliver hydrogen, methanol needs the support of an impetus as well as a moderately high temperature.
In this review, described in the June 14, 2022, online version of ACS Catalysis, the Weizmann and Brookhaven scientists concentrated on a methanol/copper framework—methanol fume bound, or “adsorbed,” onto a surface of copper—that didn’t need a high temperature. They zeroed in on “methanol deterioration,” the least difficult of the four reactions that can deliver hydrogen from methanol. Among the potential impetuses for the response, those in light of copper (Cu) are thought of as the most encouraging. This is because of a few variables, including its ideal electronic design for synergist movement and relative minimal expense and ecological wellbeing contrasted with different metals. To more readily comprehend the job of Cu, it is fundamental that researchers gain an exhaustive sub-atomic level understanding of the communication between methanol fume and Cu surfaces.
“These dynamics do not agree with what well-established models of this system tell us we should see. Instead, the evolution of methoxy coverage over time is governed by an extraordinary adsorption kinetics model.”
Baran Eren, the paper’s corresponding author and a researcher in Weizmann’s Department of Chemical and Biological Physics.
Specialists from Weizmann and the Center for Functional Nanomaterials (CFN), a DOE Office of Science User Facility at Brookhaven, took a gander at three Cu surfaces to figure out how the methanol stuck to them and how it acted. These three surface calculations are normally examined as impetuses because of the way that the Cu particles are organized, which makes them more accessible to collaborate with different mixtures electronically. The researchers discovered that methanol has an unexpected effect on all three surfaces.
“The pattern we saw across each of the three surfaces was that a ton of methanol adsorbed from the outset, then, at that point, fell to pieces into various parts that desorbed.” “After some time, the methanol inclusion arrived at a harmony point,” said Ashley Head, a specialist in the Interface Sciences/Catalysis group at the CFN and one of the paper’s creators. “We had not seen this way of behaving previously and didn’t hope to.”
To concentrate on the elements of this, the gathering utilized both infrared (IR) and X-beam spectroscopy procedures, the last option being performed at the CFN.
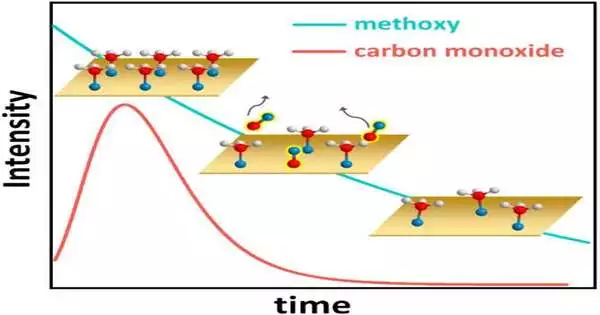
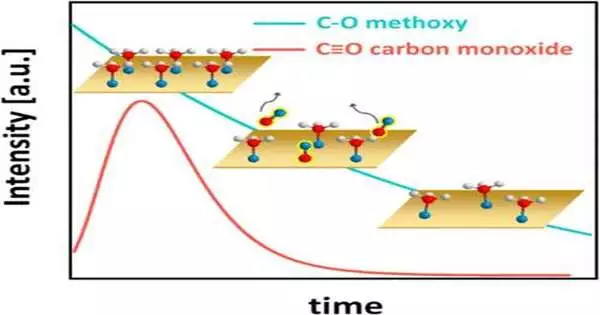
This figure portrays the amazing active way of behaving of the methanol-on-copper framework, with hydrogen molecules displayed as white circles, oxygen iotas as blue circles, and carbon particles as red circles. At first, methanol completely covers the copper (Cu) surface (upper left realistic). Every methanol particle then, at that point, loses a hydrogen molecule and structures a compound clinging to the Cu, turning into a carbon-oxygen animal type known as methoxy. The surface methoxy, thus, responds to carbon monoxide (CO), which desorbs from the surface (focus realistic, with CO inclusion over the long run addressed by the red line). The methoxy inclusion then consistently diminishes (lower right corner and blue line). In the long run, a balance of methoxy inclusion is reached (not shown).
The IR work, led by Weizmann, yielded data about what synthetic types of methanol frame on the Cu surface by estimating how the particles vibrate. The particular vibrations can be connected to explicit mixtures.
The IR information demonstrated that the methanol adsorbed onto the copper vigorously and shaped an immediate bond with the Cu, framing a compound part known as methoxy. The methoxy inclusion then slowly diminished. This conduct was seen across every one of the three surfaces, with minor fluctuations.
“These elements disagree with everything deep-rooted models of this framework say to us we ought to see,” said Baran Eren, a scientist in the Department of Chemical and Biological Physics at Weizmann and the paper’s compare creator. “All things being equal, the advancement of the methoxy inclusion with time follows a remarkable adsorption energy model.”
He proceeded, “We recommend that a brief type of methanol bound to hydrogen be the wellspring of the underlying thick methoxy layer.”
The data gathered from the IR information was affirmed at CFN, where the gathering utilized X-beam photoelectron spectroscopy (APXPS). In this procedure, X-beams empower electrons, for example, making them break free. Those catapulted electrons convey significant data. For this situation, they gave extra insight into the methanol’s conduct on the Cu surface and the encompassing circumstances. APXPS permits specialists to more effectively compute atom inclusions on surfaces than IR spectroscopy.
As time elapsed, the overabundance of methoxy was dispensed with as increasingly more hydrogen was created, leaving carbon monoxide that had de-adsorbed from the copper as a gas. The methoxy that was left arrived at a place of even inclusion — a balance point. Moreover, the energy of this cycle was impressively quicker on the more inexactly pressed Cu surface compared with the other two, which are all the more thickly stuffed.
In future work, the group intends to keep concentrating on methanol/Cu frameworks to get familiar with their elements and whether a portion of these ways of behaving could be found in frameworks other than methanol on Cu.
More information: Roey Ben David et al, Methanol Decomposition on Copper Surfaces under Ambient Conditions: Mechanism, Surface Kinetics, and Structure Sensitivity, ACS Catalysis (2022). DOI: 10.1021/acscatal.1c05933
Journal information: ACS Catalysis