As businesses across the country begin to transition to renewable energy, demand for batteries, and thus lithium, is expected to skyrocket.Yet, with a large part of the worldwide lithium supply situated beyond the United States, scientists are searching for new methods to remove it from nearby, if fairly unusual, sources like oil wastewater and geothermal salt waters.
One of the most encouraging of these extraction methods is electrochemical intercalation, a cycle in which cathodes draw lithium from, in any case, unusable water. Up to this point, the innovation had not arrived at the ideal degree of Li selectivity for very weak water assets.
Presently, analysts at the University of Chicago’s Pritzker School of Molecular Engineering (PME) have demonstrated the way that “cultivating” anodes with lithium particles can assist with expanding the host’s lithium selectivity and repulse undesirable components. Their discoveries were distributed in Nature Communications.
“It was amazing to observe these ions phase separate into two independent domains, one with only lithium and one with only sodium. It made us ponder how we may use it to improve lithium selectivity.”
Asst. Prof. Chong Liu
A significant qualification
In science, intercalation is the cycle by which “visitor” particles are brought into and put away inside a “have” material, the last option going about as a kind of sub-atomic bee colony. The cycle is likewise reversible, meaning those equivalent particles can be removed and the interaction rehashed again and again. It is the vital system behind battery-powered batteries.
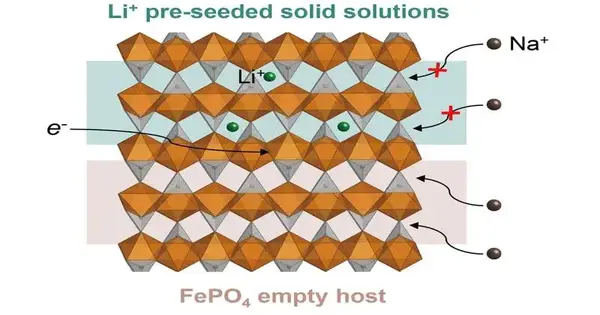
When utilized for lithium extraction, electrochemical intercalation depends on a host material — for this situation, olivine iron phosphate (a sort of gem) — that is particularly appropriate to draw in and store lithium particles. While broadly examined and one of the most amazing fit materials for the gig, olivine iron phosphate is quite flawed. Contending particles are frequently brought into the host material alongside lithium and other components, for example, sodium, which lessen the framework’s adequacy.
Liu and her group needed to comprehend what drove those co-intercalations and what happened once the two particles were put away inside the gem.

To rescue unused lithium from oil and gas wastewater, Asst. Prof. Chong Liu (right) and her group reengineer materials at the atomic level. Photographer: John Zich
Working with analysts at the University of Illinois Urbana-Champaign, Liu and her group utilized transmission electron microscopy to peer inside their host material. They found that lithium and sodium would, in general, separate whenever allowed the opportunity. This proposed that lithium and sodium particles repel each other inside the gem material, much like oil and water separate when blended, a cycle called stage division.
To affirm that way of behaving, the group created computational models as a team with scientists at the Illinois Institute of Technology.
“It was amazing to see these particles stage separate into two unique areas where one space was just lithium and one was just sodium,” Liu said. “It made us think about how we could utilize it to support lithium selectivity.”
Planting the seeds of request
Following up on their discoveries, Liu and her group contrived a framework to pre-seed their olivine with lithium. They guessed this would increase the energy boundary for sodium particles, making it harder for undesirable components to enter the host.
They found that cultivating 20 to 40 percent of the general host materials’ stockpiling locales can in fact wrinkle the selectivity to 1.6-crease and 3.8-overlap, separately. The cultivated high-Li strong arrangement stages showed areas of strength for the selectivity upgrade.
The group likewise saw that few elements, including the host morphology and deformities, added to the lithium selectivity, offering a few roads for additional exploration. Future examinations will explore the ideal cultivating conditions and host morphology to boost lithium selectivity.
“We’ve shown a viable approach to controlling the motor pathway in a host material,” Liu said. “In the event that you have some control over the lithium-sodium pathway, you have a strong switch for impacting lithium selectivity.” That acknowledgement opens an entryway for more review and, at last, a feasible framework for removing lithium. “
More information: Gangbin Yan et al, The role of solid solutions in iron phosphate-based electrodes for selective electrochemical lithium extraction, Nature Communications (2022). DOI: 10.1038/s41467-022-32369-y
Journal information: Nature Communications