The infection that causes the coronavirus, considered SARS-CoV-2, involves its spike protein adhering to and tainting our cells. The last step for the infection to enter our cells is for part of its spike protein to behave like a turn tie, constraining the host cell’s external film to intertwine with the infection.
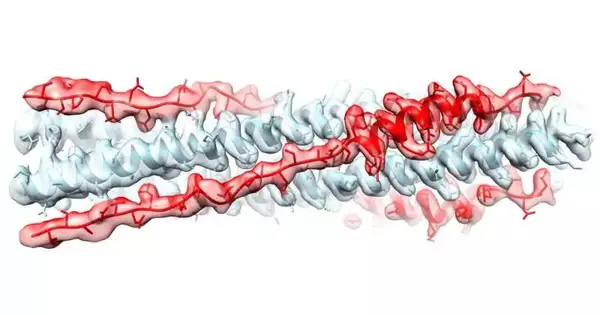
Kailu Yang, in the lab of Axel Brunger, associates at Stanford College, and teammates at the College of California Berkeley, Harvard Clinical School, and College of Finland have produced an atom in light of the contorted piece of the spike protein (called HR2), which sticks itself onto the infection and keeps the spike protein from winding.
Their exploration shows that it keeps cells from contamination even with new SARS-CoV-2 variations. Yang’s work was distributed in the Procedures of the Public Foundation of Sciences in October and will be introduced on Tuesday, February 21, at the 67th Annual Biophysical Society Meeting in San Diego, California.
“Based on the structure, we created the molecule a little longer than previously published work, and indeed, we confirmed in our fusion and infection experiments that this longer portion inhibits considerably better,”
Axel Brunger, colleagues at Stanford University,
Different coronavirus treatments have worked by attaching to the spike protein and preventing it from contaminating cells, but they have had drawbacks. For instance, bebtelovimab was a counteracting agent treatment that designated the spike protein; in any case, it didn’t function admirably against new Coronavirus variations since that piece of the spike protein has changed over the long run.
Yang and Brunger are confident that their particle, which they call the long HR2_42 inhibitor, is a lead compound to foster another sort of antiviral remedy to forestall diseases, even with new variations.
The explanation for why the longHR2_42 inhibitor might neutralize an advancing infection is that it depends on a piece of the spike protein that hasn’t changed even as different parts have.
“In the infection, there are two pieces of the spike protein that meet up, framing this pack.” “So we just took a short piece of one piece of this group, and by incorporating that little piece synthetically, it can embed itself into the spike protein and keep the infection from tainting cells,” Brunger made sense of. Past studies conducted before this coronavirus pandemic planned to make a comparable particle that would attempt to impede the disease of the SARS coronavirus; however, those previous endeavors weren’t quite as successful as the longHR2_42 inhibitor.
Brunger accepts their particle is more compelling than past endeavors because of Yang’s work deciding on an itemized construction of the bent together pieces of the SARS-CoV-2 infection, called the postfusion supposed HR1HR2 complex, so they realized longer particles would assist with impeding the spike protein from winding into the HR1HR2 complex in any case.
“We made the particle somewhat longer than recently distributed work in view of the design, and for sure, we affirmed in our combination and contamination measures that this more extended piece suppresses much better,” Brunger said.
The group is currently testing the longHR2_42 inhibitor in mice infected with SARS-CoV-2 (a collaboration with Giuseppe Ballisteri and collaborators from the University of Finland). They are confident that they will want to deliver it to individuals via inhaler so that it can reach the aviation route, which is exactly where you need to get an early disease to prevent contamination from becoming serious. “The second people start sneezing, it will be an ideal opportunity to take it,” Brunger explained.
More information: Kailu Yang et al, Nanomolar inhibition of SARS-CoV-2 infection by an unmodified peptide targeting the prehairpin intermediate of the spike protein, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2210990119