Inferable from their special porous, empty shell structures with internal, mobile centers, yolk-shell nanocrystals are reasonable for a wide assortment of uses. Tokyo Tech specialists created Yolk-shell nanocrystals with a gold center and various semiconductor shells, utilizing an original successive particle trade process.These metal-semiconductor yolk-shell nanocrystals can act as profoundly successful photocatalysts for some applications.
Yolk-shell nanocrystals are exceptional materials with interesting underlying properties like a penetrable shell, inside void space, and mobile yolk. These nanocrystals are reasonable for an assortment of utilizations, contingent upon the selection of materials utilized for their creation.
“Yolk-shell nanocrystals with a metal yolk and semiconductor shells are particularly intriguing because they can be tailored to mass transport-related applications, such as photocatalysis,”
Assistant Professor Chun-Yi Chen at Tokyo Institute of Technology (Tokyo Tech)
For instance, on the off chance that the inward surface of their shells is intelligent, yolk-shell nanocrystals can make for a solid photovoltaic gadget. A portable center can go about as a stirrer, fit for blending arrangements held inside the shell. The internal and external surfaces of the shell give a lot of dynamic destinations to responses, and the yolk-shell design’s interesting properties (a consequence of electronic cooperation and charge-move between the surfaces of the construction) make these nanocrystals ideal for photocatalysis applications. Justifiably, yolk-shell nanocrystals definitely stand out enough to be noticed by scientists around the world.
In a cooperative report distributed in ACS Applied Nano Materials, which was likewise chosen as the ACS Editors’ Choice, a global exploration group led by Associate Professor Tso-Fu Mark Chang and Assistant Professor Chun-Yi Chen at Tokyo Institute of Technology (Tokyo Tech) and Professor Yung-Jung Hsu at the National Yang Ming Chiao Tung University in Taiwan has fostered a few yolk-shell structures containing a metallic gold (Au) yolk with different semiconductor shells. Such designs have ascended in prominence overall on account of their entrancing properties, attributable to their Au centers.
“Yolk-shell nanocrystals including a metal yolk and semiconductor shells are especially intriguing in light of the fact that they can be equipped for mass vehicle-related uses, for instance, photocatalysis,” says Professor Chen.
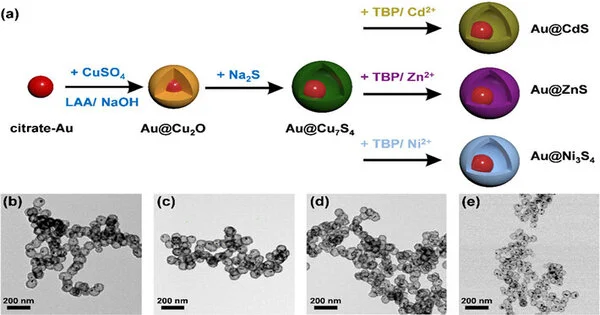
To make the nanocrystals, the scientists utilized a consecutive particle trade process. The system includes sensitive sulfidation on an Au@Cu2O center shell nanocrystal layout (where Au adds deeply and Cu2O to the shell development), followed by a dynamically controlled cation trade response that empowers change of the shell organization (i.e., Cu2O) into different metal sulfides, which are semiconductors. Four delegate yolk-shell nanocrystal tests, including Au@Cu7S4, Au@CdS, Au@ZnS, and Au@Ni3S4, were combined for examination along these lines (as displayed in Figure 1).
The presentation of these yolk-shell structures as photocatalysts was assessed utilizing X-beam photoelectron spectroscopy (XPS) and consistent state photoluminescence (PL) spectroscopy.
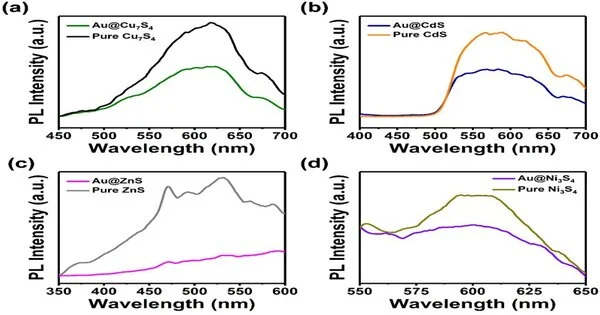
Figure 2. Consistent state photoluminescence (PL) spectra of (a) Au@Cu7S4, (b) Au@CdS, (c) Au@ZnS, and (d) Au@Ni3S. The aftereffects of their untainted partners are additionally included. Under light brightening, the nanostructures were found to have high photoluminescence (PL) movement, revealing them to be exceptionally equipped for retaining light and producing electrons and openings as charge transporters. Tokyo Institute of Technology is responsible for this image.
Utilizing XPS, the specialists tracked down the metal centers and semiconducting shells of the nanocrystals to have electronic collaborations great for photocatalysis applications. Time-settled PL spectroscopy uncovered the nanostructures to have high PL power, demonstrating high photocatalytic movement and suggesting that they were profoundly fit for engrossing light and producing electron-opening charge transporters (as displayed in Figure 2).
“In a genuine situation, the responses worked with by isolated photoexcited electrons and openings assume a part in natural cleansing, by delivering receptive oxygen species,” makes sense to Prof. Chen, portraying one situation in which their original yolk-shell photocatalysts could be utilized. These photoexcited electrons and openings can work with a huge number of responses, making yolk-shell nanocrystals relevant in many fields like natural purging, hydrogen creation, and carbon dioxide decrease.