A global group led by a University of Toronto specialist has found that a neutralizer discernible in the blood predicts extreme Crohn’s sickness and is noticeable as long as seven years preceding the illness finding.
For which simple and compelling biomarkers preceding the discovery are insufficient.A blood test could provide a fast, financially savvy, and harmless method for evaluating risk for confounded Crohn’s, which might empower preventive systems before subclinical irritation prompts persistent side effects.
At the Icahn School of Medicine at Mount Sinai in New York and a worldwide group of scientists from France and Portugal, “Our group distinguished a serological biomarker for Crohn’s illness that likewise partakes in its pathogenesis and happens a very long time before the sickness shows its full clinical range,” said Arthur Mortha, an associate teacher of immunology in U of T’s Temerty Faculty of Medicine, who composed the review with Professors Jean-Frederic Colombel and Sacha Gnjatic at the Icahn School of Medicine.
“Our team discovered a serological biomarker for Crohn’s disease that also plays a role in its etiology and develops years before the disease manifests its full clinical range,” said the researchers.
Arthur Mortha, an assistant professor of immunology
The ongoing weapons store of therapeutics that cause alleviating abatement in Crohn’s patients is great, but endures constraints. A biomarker or prescient markers to direct mediations is a clinical need, “said Mortha, who holds the Tier 2 Canadian Research Chair in Mucosal Immunology. Furthermore, our portrayal of this biomarker recommends it as a reasonable restorative objective for mediation and perhaps counteraction.”
The journal Gastroenterology distributed the discoveries today.
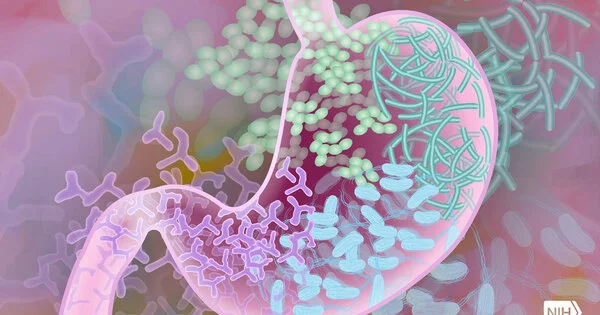
The biomarker for convoluted Crohn’s sickness is a neutralizer delivered by immune response-emitting cells in the stomach. These antibodies forestall correspondence among gastrointestinal insusceptible cells by restricting and obstructing the capacity of a protein called a cytokine. This cytokine—Granulocyte Macrophage-Colony Stimulating Factor—supports safe equilibrium in the digestive system by advancing defensive and hostile microbial resistance.
Mortha and his partners showed that in an enormous subset of Crohn’s patients, these antibodies killed the defensive impacts of the cytokine and disturbed digestive homeostasis. Those changes were distinguishable in the blood of patients years before conclusion and prompted a debilitating of the resistant framework that, over the long haul, brought about harm to the lower part of the small digestive system — a condition known as convoluted ileal Crohn’s illness.
The analysts utilized blood tests from the U.S. Branch of Defense Serum Repository to distinguish and portray the biomarker. They concentrated on examples gathered yearly for over 10 years from 220 military faculty who fostered Crohn’s and contrasted them with patients with ulcerative colitis and many solid controls.
The biomarker emphatically anticipated risk for confounded ileal Crohn’s, albeit not all patients with the counteracting agent showed precisely the same structure and seriousness of the sickness, which Mortha said features the multi-factorial nature of the condition. The biomarker was available in about a fourth of patients who had Crohn’s.
Significantly, the group also found they could save the defensive impacts of the cytokine by controlling its biochemical highlights. Designed adaptations of the cytokine with further developed biochemical highlights can be made essentially imperceptible to the antibodies, Mortha said.
“Our framework permits us to perceive how the antibodies in every patient explicitly kill the cytokine.” “We are currently designing cytokines that can get away from balance by these antibodies inside individual patients,” Mortha said. This approach could enable exceptionally customized treatments that turn around the deadening impacts of the antibodies and reestablish resistant equilibrium in the digestive system, Mortha said.
Crohn’s infection affects around 0.3 percent of the total populace, and its frequency is increasing. In Canada, which has perhaps the most elevated rate of Crohn’s, in excess of 135,000 individuals live with the condition, which can cause stomach torment, looseness of the bowels, weight loss and iron deficiency, among different side effects.
“Keeping serious areas of strength for a safe framework is fundamental for controlling the commensal organisms living in our digestive system,” said Mortha, who finished doctoral examinations in Germany and postdoctoral preparation in New York prior to setting up his lab at U of T in 2016.
“It’s staggering that our mucosal safe framework is equipped to support protection against the huge quantities of organisms in the stomach and that we’re not in complete desolation,” Mortha said. “The previous ten years have shown us a great deal of the methods of correspondence utilized by our stomach resistant cells to lay out a good arrangement at this connection point. It is currently time to bring what we have figured out how to utilize. “