Researchers at the College of Texas at Dallas have found a formerly obscure “housekeeping” process in kidney cells that launches undesirable substances, bringing about cells that restore themselves and stay working and sound.
The self-renewal process, which is fundamentally different from how other bodily tissues are thought to regenerate, helps explain how the kidneys can stay healthy for a lifetime if they are unaffected by injury or disease. In a study that was published on April 17 in Nature Nanotechnology, the researchers provided a description of the mechanism.
In contrast to the liver and skin, where cells separate to make new little girl cells and recover the organ, cells in the proximal tubules of the kidney are mitotically calm—they don’t gap to make new cells. In instances of gentle injury or illness, kidney cells really do have restricted fix capacities, and foundational microorganisms in the kidney can shape new kidney cells, but only to a limited extent, said Dr. Jie Zheng, teacher of science and organic chemistry in the School of Innate Sciences and Arithmetic and co-creator of the review.
“In most cases, kidney cells will die and cannot recover if they are severely wounded. Your kidney will simply fail eventually. This is a significant challenge in the management of kidney disease. We can only slow the progression of renal failure for the time being. We cannot readily heal the organ if it has been significantly harmed or is afflicted with a persistent illness.”
Zheng, a Distinguished Chair in Natural Sciences and Mathematics.
“In many situations, in the event that kidney cells are seriously harmed, they will bite the dust, and they can’t recover,” said Zheng, a Recognized Seat in Innate Sciences and Math. “Your kidney will simply bomb at some point or another. That is a major test of the executives wellbeing for kidney illness. Nothing remains at this point but to dial back the movement to kidney disappointment. If the organ has been severely damaged or is afflicted with a persistent disease, we cannot easily repair it.
“That is the reason finding this self-recharging system is perhaps the main finding we’ve made up until this point. With brilliant center offices and committed staff, UTD is an extraordinary spot to do such state-of-the art research.”
He stated that additional research may result in advancements in nanomedicine and the early detection of kidney disease.
A surprising finding
The scientists said their disclosure shocked them.
Zheng has been researching the biomedical applications of gold nanoparticles as imaging agents, the fundamental understanding of glomerular filtration, early liver disease detection, and targeted cancer drug delivery for 15 years. Some portion of that work has zeroed in on understanding how gold nanoparticles are sifted by the kidneys and cleared from the body through pee.
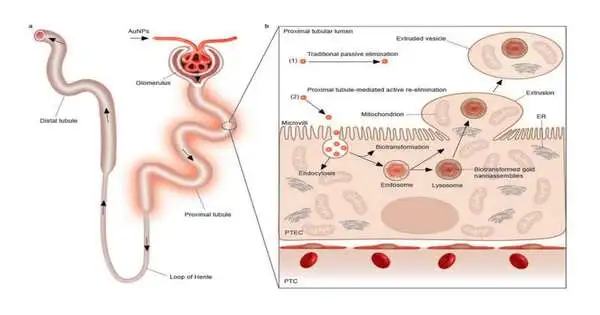
Research has shown that gold nanoparticles, by and large, pass sound through a construction in the kidney called the glomerulus and afterward travel into the proximal tubules, which make up more than half of the kidney. It has been demonstrated that proximal tubular epithelial cells internalize the nanoparticles, which then escape those cells and are excreted in urine. However, their method of escape from the cells is unknown.
In December 2021, Zheng and his science group—research researcher and lead concentrate on creator Yingyu Huang Ph.D. ’20 and co-relating creator Dr. Mengxiao Yu, research academic partner—were looking at gold nanoparticles in proximal rounded tissue tests utilizing an optical magnifying lens, yet they changed to one of the College’s electron magnifying instruments (EM) for improved goal.
“Utilizing the EM, we saw gold nanoparticles exemplified in lysosomes within enormous vesicles in the lumen, which is the space outside the epithelial cells,” Yu said.
Vesicles are little liquid-filled sacks tracked down both inside and beyond cells that transport different substances.
Yu stated, “But we also observed the formation of these vesicles outside of cells, and it was something we had not seen before.” These vesicles contained both organelles and nanoparticles.
Gold nanoparticles, lysosomes, mitochondria, endoplasmic reticulum, and other organelles that are typically found within a cell’s interior were found in proximal tubular cells that had formed outwardly facing bulges in their luminal membranes. The expelled contents were then squeezed into a vesicle that drifted off into the extracellular space.
Yu stated, “At that point, we knew this was an unusual phenomenon.” This is a novel approach to the removal of cellular contents by cells.
Another restoration cycle
The expulsion-intervened self-recharging system is essentially not the same as other regenerative cycles, like cell division, and housecleaning errands like exocytosis. In exocytosis, unfamiliar substances, for example, nanoparticles, are exemplified in a vesicle inside the cell. Then, the vesicle layer wires within the cell’s film, which opens to deliver the items to the outside.
“What we discovered is very different from what was previously known about how cells remove particles. There is no layer combination in the expulsion cycle, which disposes of old substances from ordinary cells and permits the cells to refresh themselves with new items,” Huang said. “It occurs regardless of the presence or absence of foreign nanoparticles. It’s an inherent, proactive interaction these cells use to endure longer and utilize their capabilities appropriately.”
Zheng said their discoveries open up new areas of study. In addition to the walls of arteries and the gut and digestive tract, epithelial cells, like those in the proximal tubules, can be found in other tissues.
“In the field of nanomedicine, we need to limit the aggregation of nanoparticles in the body as much as could be expected. We don’t believe that they should stall out in the kidneys, so it’s vital to comprehend how nanoparticles are disposed of in the proximal tubules,” Zheng said. “Likewise, in the event that we could figure out how to direct or screen this self-reestablishment process, we could figure out how to keep kidneys sound in patients with hypertension or diabetes.
“Maybe it could be an indicator of early kidney disease if we could develop methods to detect the signature of this process in a noninvasive manner.”
More information: Yingyu Huang et al, Proximal tubules eliminate endocytosed gold nanoparticles through an organelle-extrusion-mediated self-renewal mechanism, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01366-7