Using creature models and static cell societies, the transport of mercury particles across digestive epithelial cells can be read up for toxicology evaluations.Nonetheless, the ideas don’t dependably repeat states of the human stomach microenvironment to screen in situ cell physiology. Thus, the system of mercury transport in the human digestive tract is as yet unclear.
In another report currently distributed in Nature Microsystems and Nanoengineering, Li Wang and an exploration group in mechanical designing and regenerative medication in China fostered a stomach on-a-chip instrument coordinated with transepithelial electrical opposition (TEER) sensors and electrochemical sensors.
They proposed to investigate the unique idea of mimicking the actual digestive boundary and mirroring the natural vehicle and adsorption systems of mercury particles. The researchers reproduced the cell microenvironment by applying liquid shear pressure and cyclic mechanical strain.
Wang and the group concentrated on mercury adsorption and the actual harm brought about by the poisonous component on epithelial cells through the exhibition of electrochemical sensors subsequent to presenting them to digestive cells developing under assorted convergences of mercury blended in the cell culture medium. The group noticed the articulation and upregulation of Piezo1 and DMT1 (divalent metal carrier), both mechanosensory particle channels and iron carriers, separately on the cell surface.
Fostering a digestive model
Mercury particles are non-biodegradable and can amass in the body at low concentrations to harm significant organs. Harmful mercury particles can connect with cell reinforcement parts, DNA-fix catalysts, and proteins at the subcellular level to upset cell homeostasis and produce confused cell design and capability.
While mercury adsorption happens prevalently in the small digestive tract, elevated degrees of mercury ingestion can cause inner draining and a hole in a short time span. Prolonged exposure to low concentrations of mercury particles can also cause ongoing digestive issues.Because the digestive epithelium acts as a barrier to prevent ingested mercury from entering the circulation system and causing harmful effects, a realistic gastrointestinal model is critical for studying mercury transport components in the lab.Despite the fact that creature models and static cell societies are commonly used to focus on digestive adsorption and mercury transport, these models do not effectively restate the gastrointestinal microenvironment to replicate the living digestive tract.
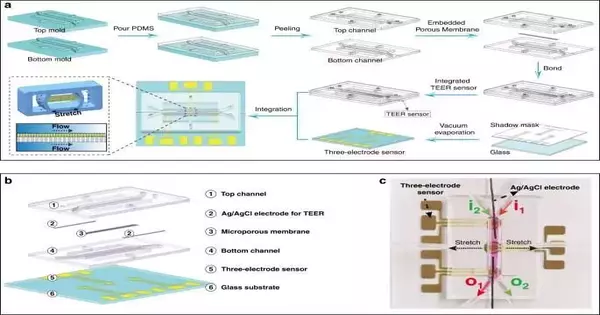
Actual property evaluation in stomach-on-a-chipa) FEA consequences of liquid shear pressure in a microchannel b) Alignment of the link between the real-time rate of the life medium into the stomach on-a-chip and the information stream via the needle siphon.Inset (I): Alignment test stage. Inset (II): A tiny picture of a liquid stream in the stomach on a chip (scale bar, 200 m). c) The FEA aftereffects of shear pressure in the stomach on-a-chip were contrasted with the genuine outcomes (n = 3). d) Quantification of the mechanical strain created in the follower stomach epithelial cells as a result of the vacuum regulator’s tension.Dark lines address direct fitting lines (y = 0.099 + 0.270x; R2 = 0.988). Inset (I): Correlation of cell masses on permeable films when extended by 3% (scale bar, 20 m). variety in the pore width of the permeable film in the span of 10 days under 1% ductile strain. RSD = 1.81% (n = 3). f) The adjustment of Youthful’s modulus of the permeable layer under 1% pliable strain in 10 days or less. RSD = 0.07% (n = 3). Credit: Microsystems and Nanoengineering (2023). DOI: 10.1038/s41378-022-00447-2
The researchers developed a stomach-on-a-chip model in collaboration with nameless sensors to continuously screen changes in transepithelial electrical opposition (TEER) during cell conductivity of mercury retention. The group recognized key highlights of the stomach through electrical estimations and immunohistochemistry studies to survey the impact on mechanosensory particle channels.
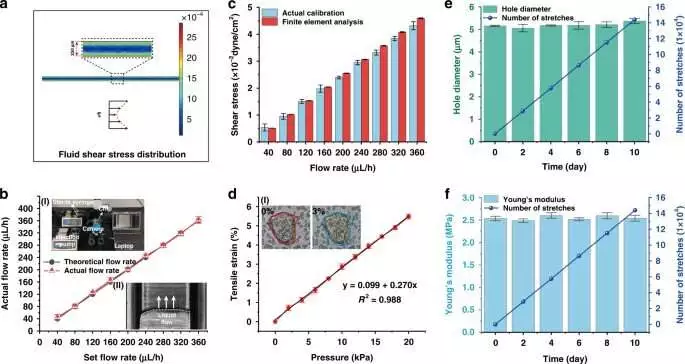
On-a-chip: qualities of the digestive epithelium in the stomach a) Tight junctional honesty of the epithelium is evaluated by estimating TEER (n = 3). b) AKP action under static (3 days, 7 days, and 21 days) and dynamic (3 days, and 7 days) societies (n = 3; *P 0.05, **P 0.01). c) (I) Confocal fluorescence perspective on a tight intersection protein (ZO-1; red) and brush line protein (EZRIN; green) in static (3 days; 21 days) and dynamic (3 days) societies (scale bar, 20 m). (II) Confocal fluorescence perspective of a cell monolayer in static (3 days; 21 days) and dynamic (3 days) culture (scale bar, 10 m).d) Normal fluorescence power examination of ZO-1 and ezrin proteins in static and dynamic societies (3 days) (n = 3; ***P 0.001). e) Refinement of normal cell level in static and dynamic societies (n = 3; *P 0.05)f) Hierarchical magnification lens perspectives on digestive villi-like designs (scale bar, 100 m).g) A SEM picture of digestive villi-like designs. h) A fluorescence-tiny view featuring the cores (DAPI) of digestive villi-like designs (scale bar, 100 m). I) A photo showing the proposed stomach on-a-chip with its checking and refined parts. Credit: Microsystems and Nanoengineering (2023). DOI: 10.1038/s41378-022-00447-2
Mathematical examination of the gadget
The research team created a chip that behaved mechanically like a living digestive tract and featured a delegate liquid stream and cyclic mechanical extending.For example, a shear pressure of approximately 0.02 dyne/cm2 created the liquid stream expected for digestive epithelial morphogenesis. In view of extra estimations on the organ chip, the group got a stream pace of 160 L/hour for the related aspects of shear pressure.
They then used limited component examination to mimic the pliable strain and stress elements in order to comprehend the effects of the boundaries on the actual properties of the instrument, remembering its pore width and their effect on cytodifferentiation by dissecting the reactant action of basic phosphatase, which is commonly used as a marker of bone and liver harm.The group noted more prominent reactant action among cells under a liquid stream and those going through mechanical burden on a chip after just seven days of cell culture.
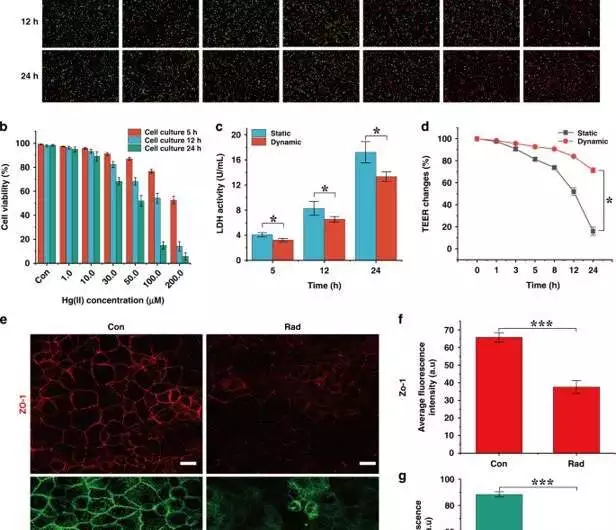
Cellular damage caused by Hg(II).a) Immunofluorescence of cells in static culture exposed to various concentrations of Hg(II) for 5, 12, and 24 hours (red: dead cells; green: living cells).b) Cell action changes in response to various Hg(II) groupings. Under low concentrations of Hg(II) (1, 10 M) (n = 3), cell activity remained above 80% after 24 hours.c) Epithelial cells were exposed to 100 M Hg(II), and LDH was measured after 5, 12, and 24 hours. The effects of LDH location revealed that the outflow of LDH in static examples was 1.3-fold higher than that in unique examples after 24 hours (n = 3; *P 0.05).d) The change in TEER rate in static and dynamic societies of cells exposed to 100 M Hg(II) for 24 hours. The TEER under unique circumstances was 4.4-fold higher than that under static circumstances (n = 3; *P 0.05). e) Confocal immunofluorescence of ZO-1 (red) and ezrin (green) proteins was observed in the absence or presence of Hg(II) (100 M) (scale bar, 20 m).(f, g) Examination of the typical fluorescence power of ZO-1 and ezrin proteins after epithelial cell injury Contrasted with the benchmark group, the fluorescence powers of ZO-1 and ezrin diminished by 1.8-fold (n = 3; ***P 0.001). Credit: Microsystems and Nanoengineering (2023). DOI: 10.1038/s41378-022-00447-2
The results highlighted the biomimetic approach and the limit in terms of cell monolayer development and separation under mechanical feeling. The stomach-on-a-chip device used biomimetic digestive villus-like designs to maintain the integrity of the tissue boundary and addressed a crucial physiologically relevant human digestive tract.
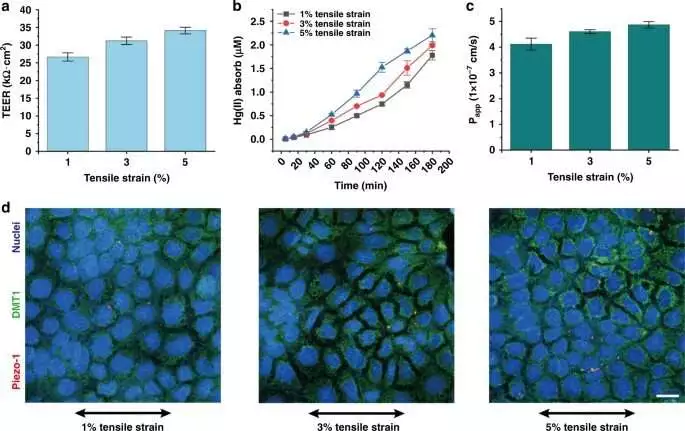
a) The TEER value of the cell monolayer on the seventh day under different mechanical stretching conditions (1%, 3%, 5%).The TEER values were 26.65 1.17 k cm2, 32.21 1.05 k cm2, and 34.10 0.93 k cm2, and the cell boundary capacity was expanded by 20.86% and 27.95% (1% mechanical feeling as the benchmark group) (n = 3). b) Changes in Hg(II) monolayer retention in digestive epithelial cells over three hours under various mechanical extending conditions (1%, 3%, 5%).Cells consumed 1.78 0.11 M, 1.98 0.03 M, and 2.20 0.14 M of Hg(II) under three mechanically extending boosts (n = 3).c) Papp esteem adjustment under various mechanical extending (1%, 3%, 5%).In comparison to and 1% mechanical stimulation, the capacity of cells to retain Hg(II) increased by 11.65% and 17.96%, respectively, under 3% and 5% mechanical stimulation (n = 3).d) The outflow of Piezo1 protein and DMT1 protein under various mechanical extending conditions (Piezo1: red; DMT1: green; core: blue) (scale bar, 20 m). Credit: Microsystems and Nanoengineering (2023). DOI: 10.1038/s41378-022-00447-2
Useful tests
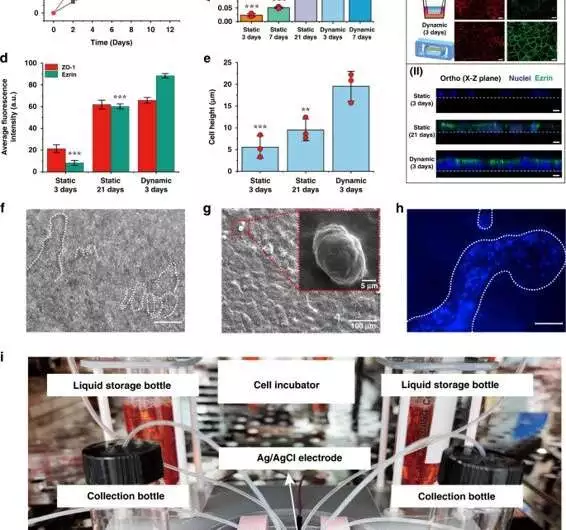
The exploration group next presented the cells to mercury under static culture conditions to grasp the course of cell demise. In addition, I noticed an increase in the cycle after increasing the Mercury focus and culture time. They noticed that the action of lactate dehydrogenase (LDH) expanded with time in the tests to show equivalent impacts of the poison across both cell societies. Nonetheless, the level of injury between the two societies varied. For example, the outflow of LDH was more prominent in the static culture compared with the unique cell societies after mercury treatment.
The researchers utilized an electrochemical sensor cluster coordinated into a stomach on-a-chip gadget to notice epithelial cell connections with mercury. They investigated the vehicle system of the component in comparison with its retention on epithelial cells and explored the outflow of key proteins, for example, Piezo1 and DMT1, in comparison with mechanosensory particle channels and dynamic iron carriers. They concentrated on the impacts of various pliable burdens on the cell boundary and noticed that an expansion in mechanical feeling prompted expanded adsorption of mercury through digestive epithelial cells, where the mechanosensory particle channels also showed a good connection.
Viewpoint
Along these lines, Li Wang and partners developed a stomach-on-a-chip gadget coordinated with transepithelial electrical resistor sensors and various electrochemical sensors to animate mercury transport in the human digestive tract in vitro. The chip elements copied an actual digestive boundary and microenvironment to notice the presence of mercury continuously. Through imaging and immunohistochemistry, the researchers observed the cell monolayer on the stomach-on-a-chip separating to form a total cell boundary. The team intends to use organ chips to advance customized drug development by understanding additional systems underlying basic human digestive illnesses.
More information: Li Wang et al, Gut-on-a-chip for exploring the transport mechanism of Hg(II), Microsystems & Nanoengineering (2023). DOI: 10.1038/s41378-022-00447-2
Sangeeta N Bhatia et al, Microfluidic organs-on-chips, Nature Biotechnology (2014). DOI: 10.1038/nbt.2989