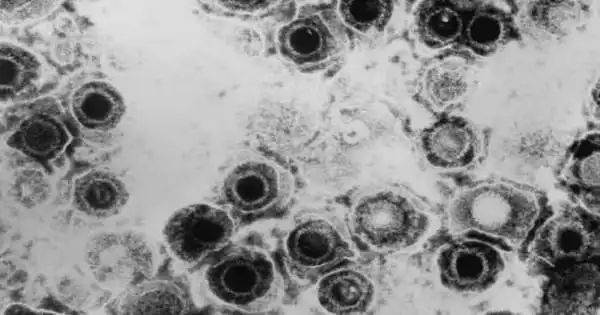
Herpesviridae is a vast family of DNA viruses that cause infections and illnesses in animals and humans alike. Herpesviruses are another name for members of this family. More than 130 herpesviruses have been identified, with some originating in mammals, birds, fish, reptiles, amphibians, and mollusks. Pseudorabies virus, the causative agent of Aujeszky’s illness in pigs, and bovine herpesvirus 1, the causative agent of bovine infectious rhinotracheitis and pustular vulvovaginitis, are examples of animal herpesviruses.
A new study published this week in mBio, an open access magazine published by the American Society for Microbiology, has paved the way for a novel method to combating herpesviruses. The study found that targeting two metal ion-dependent enzymes of human herpesviruses with two drugs, AK-157 and AK-166, can suppress virus replication. The discovery opens up new avenues for the development of anti-herpesvirus agents.
“Many people are familiar with herpes simplex viruses, but there is a family of 9 distinct herpesviruses, including cytomegalovirus (CMV), which causes significant issues in immunocompromised people, transplant recipients, and chemotherapy patients, for example. We need improved therapeutic molecules that can be employed in these really sensitive populations” Dennis Wright, Ph.D., a professor of medicinal chemistry in the University of Connecticut’s School of Pharmacy, was one of the study’s co-authors.
The majority of herpesvirus drug discovery efforts have focused on nucleoside analogs that target viral DNA polymerases. We are exploring a technique that focuses on two-metal-ion-dependent viral enzymes.
Professor Sandra K. Weller
“Right now, the treatment drugs that are available aren’t particularly successful in terms of treating all viruses, and many of them have major dose-limiting toxicities and related side effects.”
Ideally, Wright said, there would be a single medicine that would prevent the reactivation of all nine herpesviruses. Sandra K. Weller, Ph.D., a renowned professor of molecular biology and biophysics in the University of Connecticut School of Medicine, found targets that might allow this. She discovered herpesvirus enzymes that require two magnesiums in order for the virus to reproduce.
“The majority of herpesvirus drug discovery efforts have focused on nucleoside analogs that target viral DNA polymerases. We are exploring a technique that focuses on two-metal-ion-dependent viral enzymes” Weller stated.
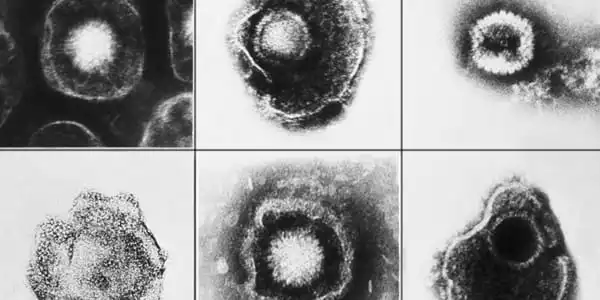
The majority of vertebrates are infected with one or more herpesviruses and will be for the remainder of their lives. The interaction between an immunocompetent healthy host and herpesviruses can be considered amicable at times. Clinically severe diseases, on the other hand, can arise when host immunity is weakened due to aging, during a stress response, during co-infections, or during neoplastic disease circumstances.
Clinical symptoms generated by original infection can differ dramatically from those caused by virus reactivation. Herpesviruses do not survive long outside of a host, therefore transmission usually necessitates close contact. The virus can reactivate in people with latent infection without generating symptoms; in such circumstances, asymptomatic shedding occurs, and people can transmit infection.
The researchers examined the capacity of a panel of chemicals to block particular 2 metal ion-dependent enzymes as well as herpesvirus replication in test tube investigations. HIV integrase inhibitors, the anti-influenza agent baloxavir, three natural products previously demonstrated to have anti-herpes simplex virus (HSV) activity, and two 8-hydroxyquinolones, AK-157 and AK-166, were among the substances evaluated.
All herpesviruses are nuclear-replicating, which means that the viral DNA is translated to mRNA within the nucleus of the infected cell. Infection begins when a viral particle comes into contact with a cell that has specific sorts of receptor molecules on the cell surface. The virion is absorbed and destroyed after viral envelope glycoproteins link to cell membrane receptors, allowing viral DNA to move to the cell nucleus. Within the nucleus, replication of viral DNA and transcription of viral genes occurs.
Infected cells transcribe lytic viral genes during symptomatic infection. Instead, a small number of viral genes known as latency-associated transcript (LAT) accumulate in some host cells. The virus can so remain in the cell (and consequently the host) indefinitely in this manner. Long-term latency is symptom-free, whereas initial infection is frequently accompanied with a self-limited period of clinical disease.
While HIV integrase inhibitors have been shown to limit herpesvirus replication, the researchers discovered that the integrase inhibitors had weak overall anti-HSV-1 effectiveness. However, the researchers discovered that 8-hydroxyquinolones have potent antiviral action against both HSV-1 and CMV, as well as the ability to inhibit one or both of the two metal ion dependent enzymes. This opens the door to the development of dual-targeting agents against herpesviruses.