Although the fundamental components of this technology are already in place, engineering organs to replace damaged hearts or kidneys in the human body may appear like something out of a futuristic film. In the expanding field of tissue design, live cells fill fake platforms to frame natural tissue. However, researchers require a reliable technique for monitoring the cells as they move and multiply in order to assess the degree to which the cells mature into tissue.
A noninvasive method for counting live cells in a three-dimensional (3D) scaffold has now been developed by researchers from the National Institute of Standards and Technology (NIST), the Food and Drug Administration (FDA), and the National Institutes of Health (NIH). The constant strategy pictures millimeter-scale locales to survey the suitability of the cells and how the cells are disseminated inside the platform — a significant capacity for specialists who make complex natural tissues from straightforward materials like living cells.
Their discoveries have been distributed in the Diary of Biomedical Materials Exploration, Section A.
“The scaffold holds things together and provides a micro-environment for whatever you want from cells. You could tune the scaffold to direct cells to behave in a specific way,”
NIST biologist Carl Simon.
To begin with, scientists made a 3D framework using an organization of polymer particles that can hold a lot of water, shaping a sort of material known as a hydrogel. After that, a type of immortal human white blood cell was embedded in the 3D hydrogel.
Cells can be extremely delicate to the climate in which they’re developed. Culture of bone cells under different conditions is required if a researcher wishes to study the growth of bone cells as opposed to breast tissue. Besides, the frameworks that house the cells are likewise produced using various materials and can fill different needs.
“The scaffold provides a microenvironment for whatever you want from cells and holds things in place.” Carl Simon, a biologist at NIST, stated, “You could tune the scaffold to direct cells to behave in a certain way.”
The team then used optical coherence tomography (OCT), a noninvasive imaging method similar to an ultrasound test but using light waves rather than sound waves.
“To decide whether a phone is alive, we investigated the optical sign made because of the movement of the organelles inside the cells,” said NIST physicist Greta Babakhanova, the first creator of the paper. By shining light through the cells, the researchers were able to determine the motion of organelles. When the organelles moved, changes in the transmitted light helped them determine whether a cell was alive or viable.
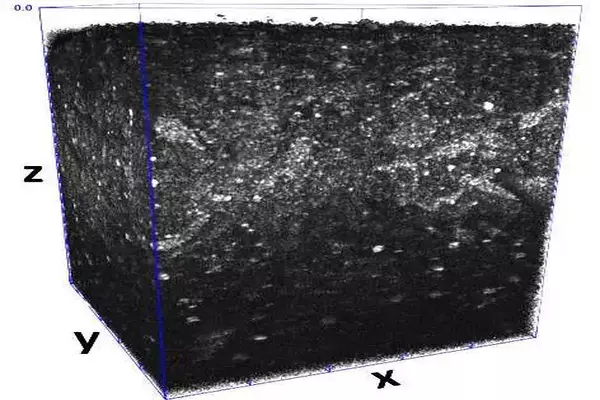
A non-invasive optical coherence tomography-captured three-dimensional volume sample of live cells in a gel. The volume is roughly 1 millimeter by 1 millimeter by 1 millimeter. Live cells are represented by the white objects in the volume sample. Credit: G. Babakhanova/NIST
The NIST method does not cut or stain samples, and it is noninvasive. The technique is additionally mark-free: in order for cells to be observed, they do not require the attachment of fluorescent molecules known as “labels.” Prior methods necessitated constant contact with the samples, which can be damaging, costly, and detrimental to the outcomes. Researchers can also complete their measurements in a fraction of the time they used to with the old method.
Additionally, the approach differs from previous ones that used flat, two-dimensional samples. The downside of the current methods is that you can quantify a specific number of cells, but you don’t have the foggiest idea where they’re found. According to Babakhanova, “we can image a one-millimeter cube of hydrogel using this method and see where the cells are located within the gel.”
According to Babakhanova, two-dimensional approaches also don’t work as well because they don’t closely mimic the three-dimensional microenvironment that cells experience in the body.
Researchers are looking into using the method to study additional properties, such as the structure of biofabricated tissue, as the next step. The OCT techniques might have the option to nondestructively measure explicit designs that develop as the tissues mature progressively as a proportion of their status for implantation,” said Simon.
Meanwhile, the technique as of now meets a neglected need in tissue design with its capacity to screen the number and plan of cells on a fake platform without dismantling and obliterating it.
More information: Greta Babakhanova et al, Three‐dimensional, label‐free cell viability measurements in tissue engineering scaffolds using optical coherence tomography, Journal of Biomedical Materials Research Part A (2023). DOI: 10.1002/jbm.a.37528





