Hydrogen, the most bountiful component in the universe, is found everywhere, from the residue occupying a large portion of space to the centers of stars to numerous substances here on the planet. Even though the atoms of hydrogen are the simplest of any element, each containing only one proton and one electron, this alone would warrant research into the element. This makes hydrogen, according to David Ceperley, a physics professor at the University of Illinois at Urbana-Champaign, the obvious starting point for developing and evaluating theories of matter.
Ceperley, additionally an individual from the Illinois Quantum Data Science and Innovation Center, utilizes virtual experiences to concentrate on how hydrogen particles interface and join to shape various periods of matter like solids, fluids, and gases. Quantum mechanical simulations, on the other hand, are expensive and necessitate quantum mechanics for a complete comprehension of these phenomena. Ceperley and his coworkers created a machine learning method that makes quantum mechanical simulations with an unprecedented number of atoms possible to simplify the process. They reported in Physical Review Letters that their method discovered a brand-new type of high-pressure solid hydrogen that previous theories and experiments failed to discover.
“We had seen signs of new behavior in our previous simulations, but we didn’t trust them because we could only accommodate a limited number of atoms. We could use our machine learning model to take full advantage of the most accurate approaches and see what’s actually going on.”
David Ceperley, a professor of physics at the University of Illinois Urbana-Champaign,
“AI ended up teaching us an incredible arrangement,” Ceperley said. “Our previous simulations had shown signs of new behavior, but because we could only accommodate a small number of atoms, we didn’t trust them. We were able to take full advantage of the most precise methods with our machine learning model and see what was really going on.”
Hydrogen particles structure a quantum mechanical framework; however, catching their full quantum conductivity is extremely challenging, even on PCs. While quantum Monte Carlo (QMC), a cutting-edge method, is capable of realistically simulating hundreds of atoms, comprehending large-scale phase behaviors necessitates simulating thousands of atoms for extended periods of time.
Hongwei Niu and Yubo Yang, two former graduate students, created a machine learning model that could accommodate a great deal more atoms than QMC by itself by being trained with QMC simulations. They then, at that point, utilized the model with postdoctoral exploration partner Scott Jensen to concentrate on how the strong period of hydrogen that structures at extremely high tensions softens.
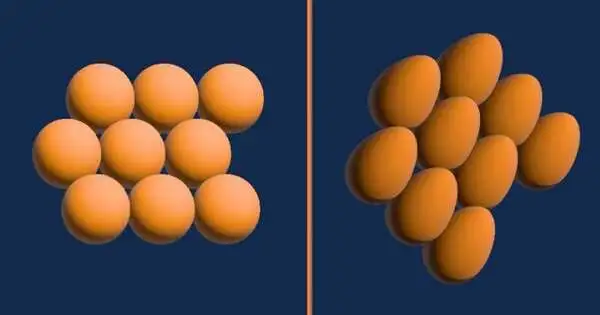
When they noticed something unusual in the solid phase, the three of them were taking measurements at various temperatures and pressures to get a complete picture. The researchers observed a phase in which the molecules become oblong figures—Ceperley referred to them as egg-like—while the molecules in solid hydrogen are typically close to spherical and form a configuration known as hexagonal close packed. Ceperley compared this configuration to stacked oranges.
According to Jensen’s recollection, “We started with the not-too-ambitious goal of refining the theory of something we know about.” Sadly, or maybe luckily, it was more intriguing than that. There was this new behavior appearing. In fact, it was the most common behavior at high temperatures and pressures, something that older theories had no inkling of.”
The researchers used data from density functional theory, a widely used method that is less accurate than QMC but can accommodate many more atoms, to train their machine learning model in order to verify their findings. They discovered that the results of standard theory were perfectly reproduced by the simplified machine learning model. The researchers came to the conclusion that, in contrast to conventional methods, their large-scale, machine learning-aided QMC simulations are able to account for effects and make predictions.
A dialogue between experimentalists and Ceperley’s collaborators has begun as a result of this work. Because it is difficult to measure hydrogen at high pressure, there are few experimental findings. Some groups have been inspired by the new prediction to revisit the issue and investigate hydrogen’s behavior in extreme conditions in greater depth.
Ceperley mentioned that our comprehension of Jupiter and Saturn, two gaseous planets primarily composed of hydrogen, will improve if we can comprehend hydrogen at high temperatures and pressures. Jensen went on to say that the “simplicity” of hydrogen makes it important to study. “Systems that we can attack should be our first target because we want to comprehend everything,” he stated. Hydrogen is straightforward, so it merits realizing that we can manage it.”
More information: Hongwei Niu et al, Stable Solid Molecular Hydrogen above 900 K from a Machine-Learned Potential Trained with Diffusion Quantum Monte Carlo, Physical Review Letters (2023). DOI: 10.1103/PhysRevLett.130.076102