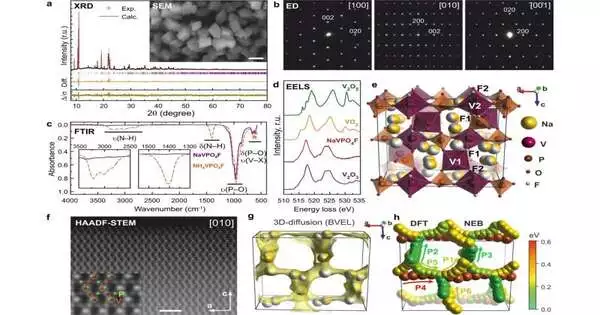
With lithium prices nearly five times higher than a year ago, scientists from Skoltech and Lomonosov Moscow State University have developed a material for sodium-particle batteries that provides an alternative to the undeniably expensive lithium-particle technology. The new material is a powder of sodium-vanadium phosphate fluoride with a specific gem structure. Utilized in the battery cathode, it gives a record-high energy stockpiling limit, killing one of the bottlenecks of the arising sodium-particle innovation. The exploration discoveries are accounted for in Nature Communications.
Lithium-particle batteries are all over: Among other things, they power compact gadgets and electric vehicles and store the energy created by wind farms to level out sporadic breeze patterns. In any case, depending on lithium alone is unsafe on the grounds that its synthetics are developing, always costly, their creation is fairly grimy, and the metal stores are unevenly distributed all over the planet. One stage lower in the occasional table, the significantly more abundant salt metal sodium lends itself as a potential alternative to lithium.
Up to this point, the sodium-particle battery technology is somewhat new, and keeping in mind that the essential design of the battery cell is something similar, various materials must be utilized for the key parts. Among them, the cathode is vital for battery quality. In their new paper, Skoltech and MSU scientists concocted another cathode material that guarantees 10%–15% preferred battery energy thickness over the ongoing strong competitor.
“Both our novel substance and the one used previously by the industry are known as sodium-vanadium phosphate fluoride, and they are composed of the identical atoms. What distinguishes them is how those atoms are organized and in what proportion they are included in the combination.”
Assistant Professor Stanislav Fedotov of Skoltech,
Both our new material and the one the business has as of late sent are called sodium-vanadium phosphate fluoride—they’re made of iotas of similar components. “What makes them different is the way those iotas are organized and in what proportion they are contained in the compound,” concentrate on co-creator, Assistant Professor Stanislav Fedotov of Skoltech, said.
“Our material contrasts well with the class of layered materials for cathodes: It gives generally a similar battery limit and more prominent strength, which translates into longer life and greater expense proficiency of the battery,” Fedotov went on. “Amazingly, even the hypothetical expectations for the contending materials miss the mark regarding the viable exhibition of our own, and this is nowhere near minor, on the grounds that the hypothetical potential is rarely completely understood.”
According to the researchers, further research into effective materials for sodium-particle batteries has led to the conclusion that they could well outperform lithium-particle gatherers in heavy electric vehicles, such as transports and trucks, as well as in fixed energy capacity at wind farms, sun-based ranches, and elsewhere.
“The higher energy stockpiling limit is only one of the upsides of this material. It likewise empowers the cathode to work at lower surrounding temperatures, which is especially important for Russia, “Fedotov said.”
Semyon Shraer, an exploration understudy at Skoltech and the lead creator of the paper, shared the beginnings of the thought behind this review: “as a matter of fact, the battery local area will in general continue with the quest for new materials either exactly—by experimentation—or with high-throughput concentrates on that test huge swaths of materials.” We approach it diversely and favor a strong state science plan. That implies we depend on hard science, utilizing the key regulations and standards of strong state science to show up the material with the wanted properties. “
He went on: “Hypothetical contemplations drove us to the essential recipe for a material that could give a high energy stockpiling limit.” “We then expected to figure out which gem design would unlock that potential.” The one we picked is known as the KTP-type system, and it comes from nonlinear optics—it’s not exactly normal for battery design. After cautious reasoning and guessing, we understood that this specific compound with that specific gem design ought to work. Then we figured out how to blend it through low-temperature particle trade. “It is right there, with its unrivaled qualities presently affirmed by a trial.”
More information: Semyon D. Shraer et al, Development of vanadium-based polyanion positive electrode active materials for high-voltage sodium-based batteries, Nature Communications (2022). DOI: 10.1038/s41467-022-31768-5
Journal information: Nature Communications