A new strategy for treating fragile X syndrome, a leading cause of autism spectrum disorders marked by an inherited repeat of certain nucleotides in the FMR1 gene’s DNA sequence, has been discovered. The research, which was carried out by staff members at Massachusetts General Hospital (MGH), has been accepted for publication in the journal Cell.
An expansion of the trinucleotide repeat CGG within FMR1, also known as Fragile X Messenger Ribonucleoprotein 1, results in FXS. FMR1 is responsible for the production of a protein called FMRP, which is necessary for the development of the brain. However, the CGG expansion in people who are born with FXS results in decreased expression of this protein, which causes developmental delays and learning disabilities as well as issues with social and behavioral issues. The problem affects 1 in every 3,000 young men and 1 in every 6,000 young ladies.
“We contemplated whether we could treat FXS by getting the trinucleotide rehash in FMR1 and reestablishing FMRP articulation,” makes sense of senior creator Jeannie T. Lee, MD, Ph.D., a sub-atomic researcher at MGH and a teacher of hereditary qualities at Harvard Clinical School. “While the business is attempting to reestablish articulation by quality treatment and quality altering, our methodology was to get the CGG rehash and reestablish protein articulation by invigorating the body’s own DNA fix systems.”
“We wondered if we could treat FXS by shortening the FMR1 trinucleotide repeat and restoring FMRP expression. While the industry tries to restore expression through gene therapy and gene editing, we took a different approach: we contracted the CGG repeat and restored protein expression by boosting the body’s natural DNA repair processes.”
Senior author Jeannie T. Lee, MD, Ph.D., a molecular biologist at MGH
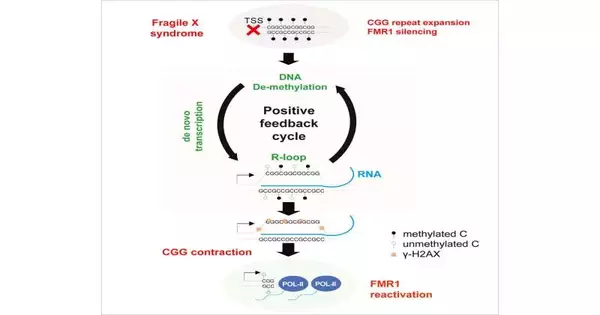
Lee and postdoctoral fellow Hun-Goo Lee, Ph.D., discovered conditions that induce a strong repeat contraction and full FMR1 reactivation by creating models from patients’ FXS cells and exposing the models to various laboratory conditions. The conditions necessitated the presence of MEK and BRAF inhibitors.
These enzymes were inhibited, which resulted in an increase in the production of unique nucleic acid structures known as “R-loops” that were formed between DNA and RNA. These structures, which cells consider to be DNA damage, prompt the cell’s repair mechanisms to address the issue. The cells’ maintenance systems then extract the extended CGG rehashes to accomplish more ordinary CGG levels, empowering cells to re-express the significant FMR1 quality.
“Since the sickness is brought about by the extended CGG rehash, getting the recurrent through R-circle development is possibly a limited-time offer of therapy,” says Lee. “The technology is now being applied to patient neurons and animal brain models.”
Sachiko Imaichi, Elizabeth Kraeutler, Rodrigo Aguilar, Yong-Woo Lee, and Steven D. Sheridan are additional co-authors.
More information: Hun-Goo Lee et al, Site-specific R-loops induce CGG repeat contraction and fragile X gene reactivation, Cell (2023). DOI: 10.1016/j.cell.2023.04.035