Even though rare earth elements like neodymium and dysprosium are essential to almost every modern technology, from smartphones to hard drives, it is notoriously difficult to separate them from the Earth’s crust and from one another.
The ability of a bacterial protein to bind to another unit of itself, or “dimerize,” when bound to certain rare earths but prefer to remain a single unit, or “monomer,” when bound to others, has been the subject of a new mechanism that has been discovered by researchers at Penn State. This allows bacteria to select between various rare earth elements.
The researchers were able to quickly, effectively, and under normal room temperature conditions separate these similar metals from one another after figuring out how this molecular handshake works at the atomic level. According to the researchers, this strategy may result in mining and recycling procedures that are both more effective and less harmful to the environment for the entire tech industry.
Joseph Cotruvo Jr., an associate professor of chemistry at Penn State and the lead author of a paper about the discovery that was published today in the journal Nature, stated, “Biology manages to differentiate rare earths from all the other metals out there—and now, we can see how it even differentiates between the rare earths it finds useful and the ones it doesn’t.” We demonstrate how these methods can be adapted for rare earth recovery and separation.
“Biology manages to distinguish rare earths from all the other metals out there—and now we can see how it even distinguishes between the rare earths it finds useful and the ones it doesn’t,”
Joseph Cotruvo Jr., associate professor of chemistry at Penn State
Cotruvo explained that rare earth elements like lanthanide metals are actually quite common, but mineralogists refer to them as “dispersed,” which means that they tend to be dispersed throughout the planet in low concentrations.
Cotruvo made the following statement: “If you can harvest rare earths from devices that we already have, then we may not be so reliant on mining them in the first place.” However, he added that, regardless of the source, it remains difficult to separate rare earths into pure forms.
“Regardless of whether you are mining the metals from devices or rock, you will still need to separate them. In theory, our method can be used to harvest rare earths in any manner,” he stated.
No different either way — and totally unique
In straightforward terms, uncommon earths are 15 components on the occasional table — the lanthanides, with nuclear numbers 57 to 71 — and two different components with comparative properties that are frequently assembled with them. The metals are frequently found together in the Earth’s crust due to their similar chemical properties and sizes. In any case, everyone has particular applications for advancements.

Because biology has already been harvesting and harnessing the power of rare earths for millennia, especially in a class of bacteria called “methylotrophs,” which are frequently found on plant leaves, in soil, and in water and play an important role in how carbon moves through the environment, the Penn State lab turned to nature to find an alternative to the conventional solvent-based separation process for rare earths. Credit: Patrick Mansell/Penn State
Conventional rare earth separation procedures necessitate the use of a significant quantity of toxic chemicals like kerosene and phosphonates, which are analogous to the chemicals that are frequently found in flame retardants, insecticides, and herbicides, as explained by Cotruvo. The partition cycle requires handfuls or even many advances, utilizing these profoundly harmful synthetics, to accomplish high-immaculateness individual uncommon earth oxides.
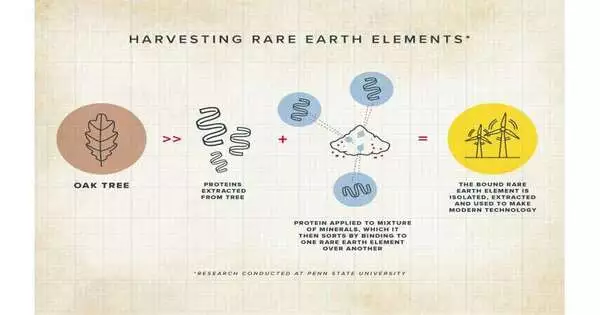
Cotruvo stated, “There is getting them out of the rock, which is one part of the problem but one for which there are many solutions.” However, once they are removed, separating multiple rare earths from one another presents a second challenge. Due to their similarity, distinguishing between individual rare earths presents the greatest and most intriguing challenge. We have engineered a natural protein we call lanmodulin, or LanM, to do just that.”
Gaining from nature
Cotruvo and his lab went to nature to find an option in contrast to the ordinary dissolvable-based detachment process, since science has proactively been collecting and bridling the force of uncommon earths for centuries, particularly in a class of microscopic organisms called “methylotrophs” that frequently are found on plant leaves and in soil and water and assume a significant part in how carbon travels through the climate.
Quite a while back, the lab disengaged lanmodulin from one of these microscopic organisms and showed that it was unparalleled — more than 100 million times better — in its capacity to predicament lanthanides over normal metals like calcium. They demonstrated in subsequent research that it was capable of separating the group of rare earths from dozens of other metals in mixtures that were too complex for conventional methods of rare earth extraction. The protein, on the other hand, had a harder time distinguishing between the various rare earths.
Cotruvo made sense of that for the new review point by point in Nature: the group recognized many other normal proteins that seemed to be the first lanmodulin yet homed in on one that was sufficiently different—70% unique — that they thought it would have a few unmistakable properties. A bacterium called Hansschlegelia quercus, which was isolated from English oak buds, naturally contains this protein.
The bacterium’s lanmodulin was found by the researchers to be highly capable of distinguishing between rare earths. Their examinations demonstrated that this separation came from the capacity of the protein to dimerize and play out a sort of handshake. The dimer (handshake) is strong when the protein binds one of the lighter lanthanides, like neodymium. On the other hand, when the protein binds to dysprosium, a heavier lanthanide, the handshake is much weaker, so the protein prefers the monomer form.
Cotruvo stated, “This was surprising because these metals are very similar in size.” With a few trillionths of a meter, or a difference that is less than a tenth of an atom’s diameter, this protein can differentiate at a scale that most of us can’t even fathom.

Penn State associate professor of chemistry Joseph Cotruvo Jr. is the lead author of a paper that describes the discovery of a novel mechanism by which bacteria can choose between various rare earth elements. This mechanism uses the ability of a bacterial protein to bind to another unit of itself, or “dimerize,” when it is bound to certain rare earths but prefers to remain a single unit, or “monomer,” when it is bound to other rare earths. Credit: Patrick Mansell/Penn State
Fine-tuning rare earth separations
The researchers collaborated with Penn State professor of chemistry, biochemistry, and molecular biology Amie Boal, who is a co-author on the paper, to visualize the process at such a small scale. The X-ray crystallography method, which enables high-resolution molecular imaging, is the focus of Boal’s lab.
The scientists confirmed that the protein’s capacity to dimerize reliant upon the lanthanide to which it was bound boiled down to a solitary amino corrosive—1% of the entire protein — that involved an alternate situation with lanthanum (which, similar to neodymium, is a light lanthanide) than with dysprosium.
Since this amino acid is essential for the organization of interconnected amino acids at the connection point with the other monomer, this shift modified how the two protein units communicated. At the point when an amino acid that is a vital participant in this organization was taken out, the protein was considerably less delicate due to its uncommon earthly personality and size. Based on the propagation of minute differences from the rare earth binding site to the dimer interface, the findings revealed a novel, natural principle for fine-tuning rare earth separations.
Their colleagues at Lawrence Livermore National Laboratory used this information to demonstrate that the protein could be tethered to small beads in a column, that it could separate the most important components of permanent magnets—neodymium and dysprosium—in a single step at room temperature, and that it could do so without the use of organic solvents.
According to Cotruvo, “this is the first time that this phenomenon has been observed in nature with the lanthanides.” “While we are by no means the first scientists to recognize that metal-sensitive dimerization could be a way of separating very similar metals, mostly with synthetic molecules,” this is fundamental science with practical results. Nature is teaching us how to do chemistry better by revealing what we are doing.
Cotruvo is of the opinion that the idea of binding rare earths at a molecular interface so that dimerization is determined by the exact size of the metal ion can be an effective strategy for achieving difficult separations.
He stated, “This is just the tip of the iceberg.” The most difficult problem of all—efficiently separating rare earths that are right next to each other on the periodic table—may be within reach with further optimization of this phenomenon.”
Based on this work, Penn State filed a patent application, and the team is currently expanding production, fine-tuning, and streamlining the protein with the intention of commercializing the procedure.
Other Penn State co-creators are Joseph Mattocks, Jonathan Jung, Chi-Yun Lin, Neela Yennawar, Emily Featherston, and Timothy Hamilton. The paper was also co-authored by Ziye Dong, Christina Kang-Yun, and Dan Park from the Lawrence Livermore National Laboratory.
More information: Joseph Cotruvo, Enhanced rare-earth separation with a metal-sensitive lanmodulin dimer, Nature (2023). DOI: 10.1038/s41586-023-05945-5. www.nature.com/articles/s41586-023-05945-5