Another examination of how the safe framework answers both more seasoned and fresher investigational antibodies for respiratory syncytial virus (RSV) will assist with advising a definitive interpretation regarding a vaccination from the lab to genuine clinical use.
The examination couldn’t come at a more vital time. The unexpected and emotional uptick in RSV cases recently demonstrated why immunization to prevent the incurable disease is so fundamentally required.Every year, RSV is responsible for 1 out of 50 pediatric deaths around the world, as per scientists at the Wilhelmina Kids’ Clinic in the Netherlands, where clinical specialists have as of late finished a concentrated study on RSV.
The majority of those deaths occur in babies who are too young to fight the viral illness.However, as data from the World Health Organization shows, the irresistible specialist is also a foe of slightly older, more seasoned adults, making the development of a viable immunization a clinical necessity to prevent pointless deaths at the far reaches of human life.
“The amplitude and quality of the serological and B cell responses were examined across time points and vaccinations. RSV A and B neutralization was measured.”
Dr. Lauren Chang
“There is right now no authorized antibody for respiratory syncytial infection,” explains Dr. Lauren Chang, who, with a huge group of partners at the Immunization Exploration Focus of the Public Foundation of Sensitivity and Irresistible Illnesses in Bethesda, Maryland, created a complete report examining potential RSV antibodies.
latest RSV immunization applicants’ capability by evoking antibodies against the alleged combination protein of RSV, reports Chang, lead creator of the new exploration, which included researchers at AstraZeneca’s Antibody and Safe Treatments division in Gaithersburg, Maryland.
The microbe utilizes its combination protein to taint human cells and start the fast fountain of harmful events that result in all-out disease.
The point of the exploration was not exclusively to look at investigational antibodies, but to explain how various sorts of RSV immunizations draw on the safe framework. Not planned as a point of the investigation is the look into the future, revealing the type of RSV immunization with the best chance of winning administrative approval in the end.
“Here, we surveyed the impact of RSV combination protein conformity on B cell reactions,” Chang added, alluding to her group’s discoveries, which are distributed in the journal Science Translational Medication.
The combination protein, otherwise called the F protein, is basically liable for joining with and entering host cells, tainting them. The F protein is likewise responsible for the ensuing cell-to-cell spread, or “syncytium development,” during RSV disease.
Syncytium development implies the combination of numerous RSV-tainted cells glommed together; thus, the name “respiratory syncytial infection.” RSV is divided into two subtypes, An and B, whose strengths substitute during various plague seasons.
“We analyzed the size and nature of the serological and B-cell reactions across time points and antibodies,” Chang proceeded. “We estimated RSV An and B balance,” Chang wrote, referring to immunizer action on rare strains of the disease.However, the group dug deeper and focused on different types of RSV immunizations and how each had the potential to connect with B cells and elicit immune responses.
It was a thorough report for a complex viral disease. The microbe has a liking for epithelial cells in the lungs and different pieces of the pneumonic tree. Because of the disease’s complexity, babies and the elderly may have difficulty locating the contamination, even if shaking is possible.
Until the alleged “tripledemic” lately, very few individuals knew about RSV, except if they or somebody close had managed the respiratory disease, specialists say.
Yet, the startling combo of influenza, coronavirus, and RSV not only made RSV an easily recognized name, it also acclimated wide areas of the worldwide populace to one more occasional respiratory danger. According to the World Wellbeing Association, RSV will generally contaminate the lower respiratory tract and is the cause of a large number of diseases each year.
Research by Chang and her partners at the Immunization Exploration Focus of NIAID shows up in excess of 50 years after the main endeavors to foster an RSV antibody.
Attempts to create an RSV date back to the 1960s, but early efforts were futile because they were harmed by an ineffective immunization.According to the WHO, researchers at the time developed an RSV vaccine that caused a serious—and in two cases fatal—fierce lung reaction during the main normal RSV disease after immunization of RSV-gullible babies.
Concerns about the broken vaccination system “have hampered improvement of alternative RSV immunizations for a long time,” experts at the WHO stated in an explanation.By the by, lately, expanded comprehension of the science of RSV and related mechanical advances have brought about various antibody applicants now in the clinical turn of events, some of which might get administrative endorsement soon.
Chang and his colleagues were able to analyze immunization types because of the growing number of investigational antibodies over time.
The researchers investigated how immunization applicants brief the rise of antibodies against the RSV combination protein.Prior immunizations depended on the inactivated post-combination type of the protein, while fresher competitors will generally utilize a settled form of the dynamic pre-combination compliance. In any case, not many tests had immediately analyzed the two antibody systems—at least, not until recently.
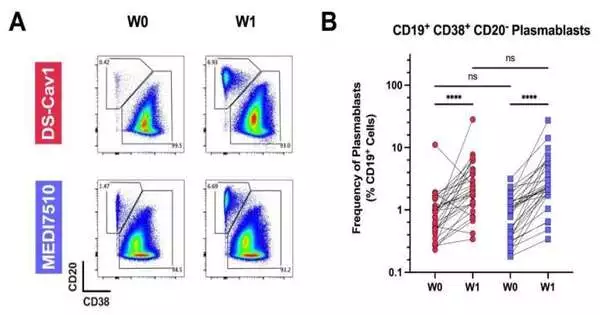
Chang and partners completely analyzed the immunizer-creating abilities of the pre-combination RSV sort of antibody and the post-combination type. The analysts examined antibodies in serum tests from people who got either the DS-Cav1 pre-combination antibody or the MEDI7510 post-combination vaccination.
Despite the fact that both antibodies elicited antibodies against the combination protein, antibodies from the pre-combination vaccination killed both the An and B subtypes of RSV in culture.Chang, and this is what partners anticipated in terms of immunization types, a prefusion-settled RSV F antibody gets B cell reactions with greater expansiveness and power than a post-combination F antibody.
Further work showed this was mostly on the grounds that the prefusion immunization evoked antibodies that can tie to extra locales on the F protein that were absent in the post-F structure.
In a related Center article, likewise distributed in Science Translational Medication, Dr. Mark Peeples of Cross Country Kids’ Clinic in Columbus, Ohio, remarks on the exploration by Chang and her partners.
Peeples noticed that the new discoveries about immunizer restriction may likewise apply to other infections, like SARS-CoV-2. Chang and his colleagues’ findings “are significant not only for RSV and Coronavirus antibody development, but also for the advancement of immunizations against other wrapped infections,” he concluded.
More information: Lauren A. Chang et al, A prefusion-stabilized RSV F subunit vaccine elicits B cell responses with greater breadth and potency than a postfusion F vaccine, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.ade0424
Mark E. Peeples, Next-generation RSV vaccines avoid flipping out, Science Translational Medicine (2022). DOI: 10.1126/scitranslmed.ade9984