Two awful realities about chemotherapy: It can hurt sound cells as well as dangerous ones, and numerous remedial targets stay inside disease cells, making them harder to reach.
Binghamton College biomedical specialists are among those investigating the utilization of cell-inferred nanovesicles to convey restorative specialists to the inside of malignant growth cells with better exactness and productivity. The little sacks of proteins, lipids, and RNA that cells discharge as a technique for intercellular correspondence could be changed to convey drugs.
“These nanocarriers have a few fantastic properties,” said Yuan Wan, an associate teacher in the Thomas J. Watson School of Designing and Applied Science’s Branch of Biomedical Designing. “For instance, they can be reaped from human cell strains, so the insusceptible reaction is extremely low. That takes into consideration ideal biocompatibility, so they dodge resistance leeway and have a lengthy blood half-life. The ideal opportunity for flow around the body is perhaps 45 seconds, so the medication-stacked nanovesicles can securely make a trip to the growths commonly, and the medications have more opportunities to be taken up by disease cells compared with drugs uninhibitedly brought into the body.
“People commonly use nanocarriers known as polymer-decorated liposomes, which have already been approved by the FDA. However, they are not perfect, as they do not have any cancer-targeting effect and may have very severe immunogenicity issues [triggering an immune response].”
Yuan Wan, an assistant professor in the Thomas J. Watson College of Engineering and Applied Science’s Department of Biomedical Engineering.
“A lot of exemplified medications can be very well safeguarded and held by the nanovesicles’ lipid films. When disease cells take up these nanovesicles, high medication fixations in the growth microenvironment really kill malignant growth cells. In correlation, free medications can diffuse rapidly and, afterward, are cleaned from the body. Just an exceptionally minuscule amount of medication arrives at the growths, making treatment viability extremely low. You can expand the portion; however, a higher portion likewise brings about high orderly poisonousness.”
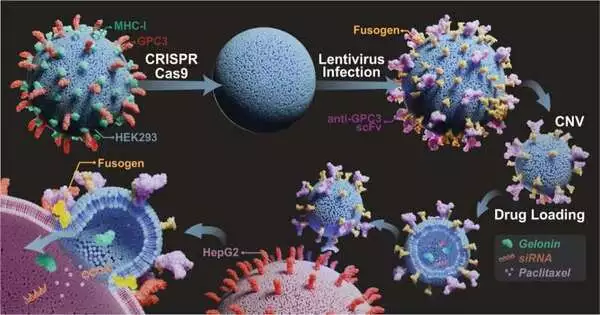
In their new review, distributed in Nature Correspondences, the Binghamton group explored different avenues regarding focusing on moieties and designed viral fusogens, which are proteins that work with malignant growth by focusing on and combining cell films.
By distinguishing overexpressed or disease-specific antigens that happen in threatening cells and focusing on moieties and fusogen-co-prepared nanovesicles, typified drugs are infused into malignant growth cells while letting sound cells be.
“Individuals broadly use nanocarriers known as polymer-embellished liposomes, and they are now supported by the FDA,” Wan said. “Yet, they are somewhat flawed, in light of the fact that they have no disease focusing on impact and may have exceptionally serious immunogenicity issues [triggering a reaction by the resistant system].”
In 2021, Wan embraced the examination of plasma-determined extracellular vesicles to analyze whether single aspiratory knobs found in human lungs are harmless or dangerous. Different strategies for deciding danger either take excessively long or are more obtrusive.
By utilizing that information, this ebb and flow, however, separates exploration outfits nanovesicles so they work for ourselves and are explicit in what they influence. Preferably, specialists could set up these focusing on moieties and fusogen-co-prepared nanovesicles for use in more secure immunization conveyance and hereditary design.
With respect to what’s straightaway, Wan said, “We want to show their treatment viability in huge creature models and exhibit that we needn’t bother with a lot of these vesicles since we’ll have the film combination capability. Assuming you bring down the quantity of vesicles and medications you want, you bring down the expense of the treatment and the incidental effects.”
More information: Lixue Wang et al, Bioinspired engineering of fusogen and targeting moiety equipped nanovesicles, Nature Communications (2023). DOI: 10.1038/s41467-023-39181-2