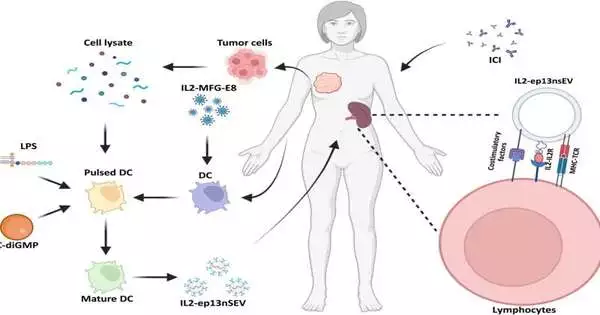
Immunotherapies don’t work for breast cancer. As a result, bioengineers and oncologists are working on a variety of therapeutic approaches to overcome this obstacle. Kerui Wu and colleagues from the departments of cancer biology, translation biology, and breast surgery in the United States and China engineered active immunotherapy to produce smart nanovesicles for individualized treatment, as reported in a new Science Advances article. Interleukin-2 (IL2), a bioactive protein bound to the membrane, was used as an anchor by the research team to accomplish this. made by a sort of T lymphocyte) to keep up with enhancing and immune system microorganism-advancing co-stimulatory factors on dendritic cell-inferred little extracellular vesicle surfaces.
Dendritic immune cell-bound major histocompatibility complex-bound antigens were observed in the nanovesicles. They observed how the surface-bound IL2 directed nanovesicles toward lymphoid organs associated with immune cells in order to activate lymphocytes with similar immune receptors following administration. The vesicles named “IL2-ep13nsEV” prompted areas of strength for a response in vivo to protect roughly half of mice embedded with patient-determined xenografts while sharpening disease cells to resist designated spot inhibitor treatment to forestall growth repeat. Preclinical models show that the findings can be used to treat and prevent metastatic breast cancer, which has applications for a wide range of cancers.
Smart vesicles that have been nanoengineered
Clinical oncologists and bioengineers are working to develop a number of new immunotherapeutic to improve the clinical outcome of particular cancers. Immune checkpoint inhibitors based on monoclonal antibodies, for instance, are extremely effective in treating patients with leukemia, melanoma, and lung cancer. Immune checkpoint inhibitor therapies, on the other hand, are frequently met with acquired resistance and relapse in the majority of patients.
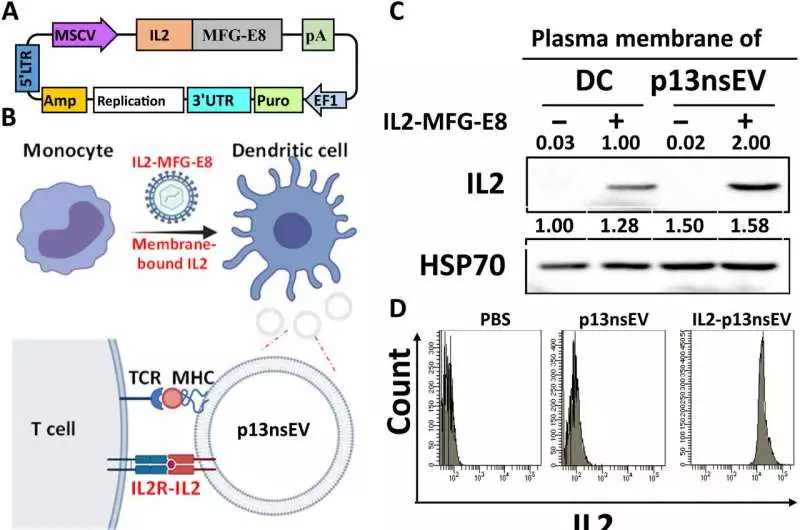
Induction of costimulatory factors on the surface of p13nsEV and engineering of membrane-bound IL2 A) The lentiviral plasmid’s structure, which allows for the ectopic expression of the IL2-MFG-E8 fusion protein on the p13nsEV membrane. B) The schematic representation of the proposed method for gluing IL2 to the surface of p13nsEV in order for it to bind to the IL2 receptor on T cells A Western blot analysis of IL2 and HSP70 expression was used to isolate the membrane fractions of p13nsEV and DCs prior to and following IL2 expression. D) FC of p13nsEV surface IL2 expression with or without surface IL2 expression (E and F) p13nsEV/PlamGFP and IL2-p13nsEV/PalmGFP were cocultured with Vybrant DiD-labeled T lymphocytes. Fluorescent microscopy (FC) and electron microscopy (E) were used to examine and quantify the interaction between T cells and sEVs after 12 hours. In each group, N is three. The unpaired Student’s t test was used for the comparison. * P 0.05, ***P 0.001, ****P 0.0001. Scale bars, 100 m. Long terminal repeat, 5’LTR; 3’UTR, the untranslated area; Mean fluorescence intensity, or MFI. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade0625
Cancer resistance can result from a low mutation burden. As a result, a number of active immunotherapies and inhibitors are designed to increase T-cell anti-tumor activity and generate immune cells that target tumors. However, the controlled release of inflammatory cytokines can have a life-threatening effect on the translational impact of such therapies as engineered chimeric antigen T cells.
Bosom disease cells are more impervious to immunotherapy because of their low transformation rate when compared with other malignant growth types. In order to produce enhanced active immunotherapy, Wu and colleagues used immunotherapy with lipopolysaccharides and stimulator interferon gene agonists to increase the expression of a variety of co-stimulators on the surfaces of nanovesicles. Along with bioactive IL-2 and enriched costimulatory factors, the team loaded the vesicle with major histocompatibility complex-presenting antigens derived from tumor lysates. The targeted lymphocytes and T cells of this nanoengineered smart vesicle were sought for individualized breast cancer treatment.
Engineering co-stimulatory factors on the smart nanovesicle
Wu and colleagues developed active immunotherapy by engineering natural nanovesicles secreted by dendritic cells to induce specific antitumor effects via lymphocyte activation in an immune-suppressive environment. They did this by engineering co-stimulatory factors into the smart nanovesicle. In order to elicit immunity, the dendritic cell-derived nanovesicles maintained essential functionality. To bioengineer, the team isolated the nanovesicles from human primary cell lines and a mouse dendritic cell line, giving them the names “p13nsEV” and “IL2-ep13nsEV” during the study. They checked the reasonability of the builds and decontaminated them, followed by electron microscopy to picture saucer-formed proteins communicating significant his compatibility loci I and II on the surfaces.
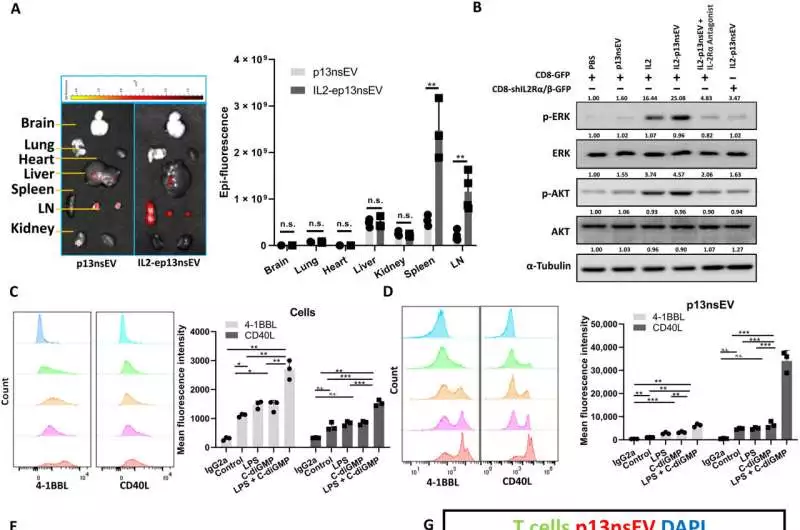
T cells become more immune to cancer cells as a result of IL2-ep13nsEV. (A) The ExoGlow was used to label the sEVs before they were injected into mice’s tail bases. The IVIS spectrum was used to measure the vesicle’s organ distributions six hours later. The signal strength in various organs was compared using two-sided unpaired t tests. In the LN groups, N = 4, while in the other groups, N = 3. B) The T cells that were subjected to sEV treatment underwent Western blotting. C) Various combinations of cytokines were used to stimulate the DCs. Then, 4-1BBL- and CD40L-positive DCs or p13nsEV were disconnected from treated DCs. (D) were examined using FC, and the two-tailed unpaired t test was used to compare the mean fluorescence intensity. N = three for each group. E) OVA (250 g/ml) was pulsed into DCs with or without IL2-MFG-E8, LPS/C-diGMP enhanced costimulatory factors, and p13nsEV was purified. CD8 lymphocytes from OT-I mice were then cocultured with various kinds of sEVs or beat DC for 5 days. In order to examine the activated IFN-+ CD8 T cells, FC was carried out. The baseline signal was determined using an isotype IgG1 control. The populations were compared using unpaired two-tailed t tests. N = three for each group. (F) The CD8 lymphocytes from (E) were cocultured with B16-OVA cells communicating GFP at a 10:1 proportion. The Zombie Aqua dead cell labeling dye was used to measure the number of cancer cells that had died among GFP+ cells. The populations were compared using unpaired two-tailed t tests. N = three for each group. G) After injecting Vybrant DiD-labeled p13nsEV into mice, the LNs were removed six hours later, and the sections were examined under a microscope and stained for lymphocyte markers. Scale bars, 100 m. n.s., P 0.05, *P 0.05, **P 0.01, ***P 0.001. 4′,6-diamidino-2-phenylindole, or DAPI. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade0625
The researchers infused the nanovesicles into mice through the tail base and imaged them across different collected organs to investigate their take-up. Nanovesicles carrying neoantigens to immune cells are likely responsible for widespread circulation and infiltration of other immune organs, as Wu and his colleagues observed significant infiltration of the constructs in secondary lymphoid organs.
The team tested the ability of bioengineered nanovesicles to enhance cancer cell-specific killing of cytotoxic T lymphocytes in a mouse model and tracked the tumor growth of mice treated with such constructs. Effects of the nanovesicles on breast tumor growth in a mouse model They found that mice treated with nanovesicles had the lowest tumor burden after four weeks. Natural killer cells increased in treated mice as the researchers tested whether these constructs could mobilize T cells into tumor lesions. They noticed the meaning of nanovesicle communications with explicit white blood cells, for example, Compact Disc 4 and CD 8, to stifle disease.
The nanovesicles can cooperate with safe designated spot inhibitors to upgrade the impact of the last system during bosom malignant growth treatment. While nanovesicles empower cancer development concealment in resistant virus mouse models, they also help the technique for safe designated spot restraint. To elicit reactivity in the immune system, the two approaches worked well together. For instance, while the immune checkpoint inhibitors maintained their viability to completely eradicate cancer cells, nanovesicles produced lymphocytes that were specific for cancer cells. When compared to either method on its own, the number of tumor-infiltrating lymphocytes and active T cells in the spleen was higher when both methods were used together.
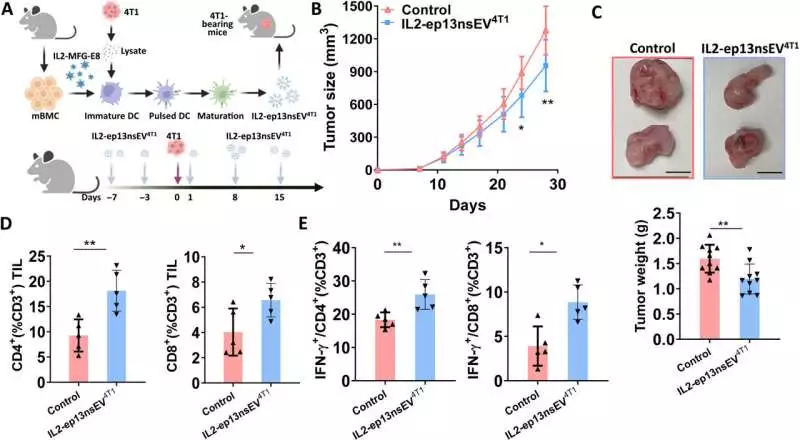
Immune cold breast tumors are affected by IL2-ep13nsEV. A) A procedure diagram for the experiment After loading tumor cell lysate from 4T1 into mBMDC and expressing IL2-MFG-E8, DC differentiation and stimulation occurred. The 4T1 tumor-bearing BALB/c mice were treated with the sEVs that were isolated from the DC. A total of 1.0 104 malignant growth cells were infused into the mammary fat stack of 6- to 8-week-old female BALB/c mice. The mice received intramuscular injections of fifty micrograms of sEVs three, seven, and fifteen days before and after the implantation of tumor cells, respectively. The control group used PBS. ( B) Cancer development on the mammary fat cushion was observed by estimating the growth size with a caliper. The sizes of the tumors were compared using the two-tailed unpaired t test. In each group, N = 10 (C). The tumor weight on day 28 represents the end point for the two treatment groups. The tumor weight was compared using an unpaired two-tailed t test. In each group, N equals 10. Scale bars, 1 cm. (D) CD4+ and CD8+ TIL among CD3+ cells were measured by FC for each group, and the percentage of TIL was compared using the two-tailed unpaired t test. In each group, N = 5 (E) FC examined the IFN-+/CD4+ and IFN-+/CD8+ ratios among CD3+ cells in dissociated splenocytes. The two-tailed unpaired t test was used to compare the percentage of cells. In each group, N is five. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade0625
Preclinical studies using a humanized patient-derived xenograft
Wu and colleagues tested the anti-tumor effect of Nano constructs using a patient-derived xenograft and combined the approach with immune checkpoint inhibition as before. They noted that the combined technologies inhibited tumor growth in humanized mice more effectively. The results of the histochemistry showed that the active immunotherapy vesicle treatment made more T lymphocytes enter the tumor.
While patients with advanced breast cancer receive immune checkpoint inhibitors, the majority of patients with early-stage cancer undergo surgery as a treatment option. Nevertheless, patients may experience recurrence, which can result in death from breast cancer.
To prevent future disease recurrence, Wu and colleagues recommend personalized active immunotherapy with nanovesicles in such situations. On a mouse model, they tested this theory and found that an individualized treatment plan could significantly reduce disease recurrence. After 8 weeks of administration, they found that the drug had no effect on the animals’ weight, liver function, or hyperactivation of immune cells.
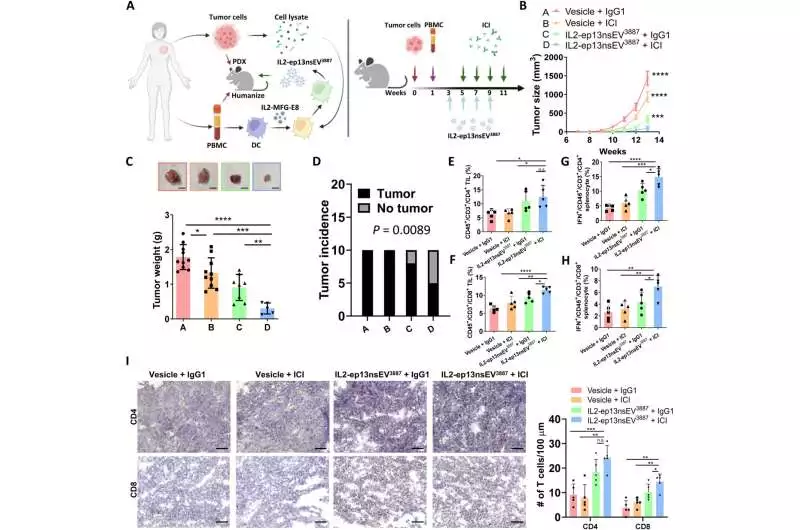
Humanized PDX mice’s response to combination therapy A) A procedure diagram for the experiment After loading PDX tumor cell lysate with IL2-MFG-E8 expression into human monocyte-derived DC, DC differentiation and stimulation took place. The IL2-ep13nsEV was confined to the DC and utilized as dynamic immunotherapy to treat adapted PDX mice. At weeks 3, 5, 7, and 9 following PDX implantation, fifty micrograms of sEVs were administered. The ICI treatment was given at 20 mg/kg intraperitoneally at weeks 5, 7, 9, and 11 after cancer implantation. ( B) Cancer development on the mammary fat cushion was observed by estimating the growth size with a caliper. The tumor sizes were compared at various points in time using the two-tailed unpaired t test. As a vesicle control for IL2-ep13nsEV3887, the sEVs that were purified from DC pulsed with the lysate of a healthy mammary fat pad were utilized. The anti-PD1 treatment was controlled with isotype IgG1. In each group, N = 10 (C). The growth weight toward the end point for the four treatment bunches is estimated and analyzed by a two-tailed unpaired t test. In each group, N equals 10. Scale bars, 1 cm. (D) The chi-square test was used to compare the tumor incidence of the four groups. E and F) The tumors were separated, and the hCD45+/hCD3+, CD4+ (E), and CD8+ (F) TIL were measured by FC and compared using an unpaired t test for each group. In each group, N = 5 (G and H) FC was used to examine the CD4+ (G) and CD8+ (H) hCD45+/hCD3+/IFN-+ cells that were present in dissociated splenocytes. The two-tailed unpaired t test was used to compare the percentage of cells between the various groups. In each group, N = 5 (I). The two-tailed unpaired t test was used to compare the intratumoral CD4 and CD8 TIL. 50 m scale bars In each group, N is five. n.s., P 0.05, *P 0.05, **P 0.01, ***P 0.001, ****P 0.0001. Credit: Science Advances (2023). DOI: 10.1126/sciadv.ade0625
Outlook
In this manner, Kerui Wu and the research team conducted a laboratory investigation into breast cancer intervention, a kind of cancer that occurs most frequently in the United States. Despite the fact that non-metastatic malignant growths can be treated with a medical procedure and chemotherapy, roughly 22% of patients with bosom disease experience a repeat in 10 years or less.
Most existing treatments fall behind in saving patients with a 10-year endurance rate by around 13%. Consequently, improved treatment is necessary to treat patients in advanced stages and prevent disease recurrence. The study’s findings look promising for treating tumors that are resistant to immune checkpoint inhibition as well as other types of cancer.
More information: Kerui Wu et al, Engineering an active immunotherapy for personalized cancer treatment and prevention of recurrence, Science Advances (2023). DOI: 10.1126/sciadv.ade0625
Wouter Scheper et al, Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers, Nature Medicine (2018). DOI: 10.1038/s41591-018-0266-5