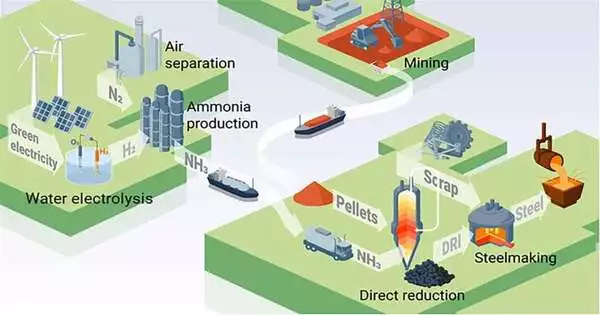
Everyone talks about hydrogen when it comes to sustainability and green steel. However, current methods of storing and transporting hydrogen necessitate high pressures and low temperatures, which are both expensive from an energy and financial standpoint. Ammonia is well known as a reliable hydrogen carrierinability and green steel. However, current methods of storing and transporting hydrogen necessitate high pressures and low temperatures, which are both expensive from an energy and financial standpoint. Ammonia is well known as a reliable hydrogen carrier. Ammonia is a strong candidate to replace hydrogen because, as demonstrated by Yan Ma and colleagues, it can be used to directly reduce iron in addition to carrying hydrogen.
For sustainable iron and steel production, Max Planck materials scientists use ammonia. Their most recent research has been published in the journal Advanced Science.
With 7% of all CO2 emissions coming from steel production, it is currently the single biggest contributor to global warming. Scientists in the sector are closely examining hydrogen-based ironmaking techniques as environmentally friendly alternatives to carbon reducers in an effort to reduce these emissions.
“Our goal was to see if ammonia could be used directly to reduce iron ores without first breaking it down into hydrogen and nitrogen. By avoiding the cracking process, overall expenses can be reduced by 18%. Furthermore, we investigated how ammonia as a reduction agent influences the characteristics of reduced iron.”
Dr. Yan Ma, group leader at MPIE and first author of the publication.
Although hydrogen-based direct reduction of iron ore shows promise, researchers still have a significant obstacle to overcome: in order to make the entire steelmaking process climate friendly, the energy and hydrogen needed for it must be produced in environmentally friendly ways. Since current methods of storing and transporting hydrogen require high pressures and low temperatures, which are both energy- and cost-intensive, there is a shortage of green hydrogen on the market.
Researchers at the Max-Planck-Institut für Eisenforschung (MPIE) used ammonia as a hydrogen carrier and an iron reductant to meet this challenge. They evaluated the costs and process characteristics of the novel method, compared the iron and steel made using ammonia-based direct reduction to those made using hydrogen-based direct reduction, and then published their findings in the journal Advanced Science.
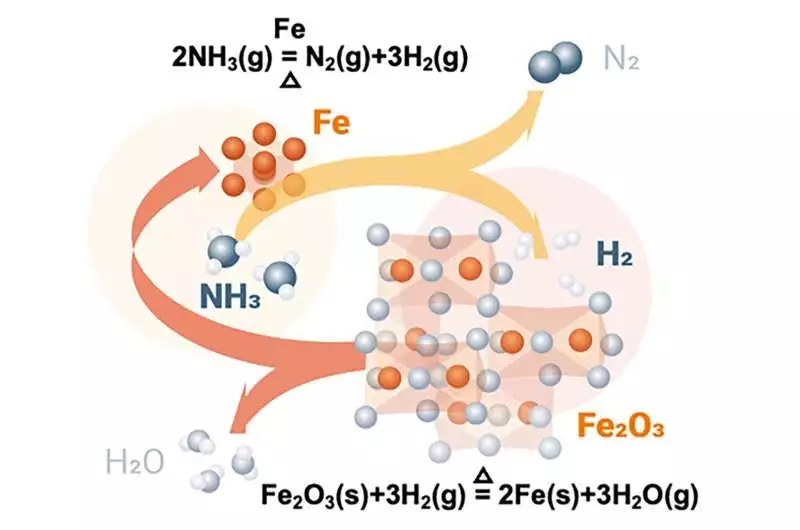
Iron oxide autocatalytic reduction by hydrogen released from ammonia cracking during the direct reduction process. Credit: T. You, Max-Planck-Institut für Eisenforschung GmbH
Ammonia has various advantages as a straight reductant.
Worldwide demand for hydrogen is rising, but the gas is difficult to store and move because of its low volumetric energy density. As a result, it must either be stored at extremely low temperatures or under high pressure. Thirty percent of the chemical energy that hydrogen provides is expended in these circumstances. Ammonia, on the other hand, is already a widely used commodity with well-established logistics and a reputation as a superior hydrogen carrier with affordable liquefaction.
“We set out to investigate whether iron ores could be reduced directly by ammonia without first being cracked into hydrogen and nitrogen. The overall costs can be cut by 18% if this cracking process is avoided. Additionally, we looked at how the reduction agent ammonia affects the reduced iron’s properties, says Dr. Yan Ma, group leader at MPIE and first author of the study.
In a laboratory-scale reactor where iron ores are transformed into so-called sponge iron, the researchers added ammonia. The weight and gas composition were measured during this process using thermogravimetry and mass spectrometry, which revealed the degree of reduction and the beginning of ammonia decomposition. “An autocatalytic reaction is used to carry out the ammonia-based direct reduction. We contrasted its kinetics with that of direct reduction using hydrogen. Both exhibit comparable traits and produce the same level of metallization. Nitrides, which can shield sponge iron from corrosion and make it easier to handle, are formed during cooling in ammonia as opposed to hydrogen-based reduction, says Ma.
During the subsequent melting procedure, which is required for downstream processing, the nitride phase can be entirely dissolved and removed. Additionally, nitrogen, the other byproduct of ammonia decomposition, can function as a heat carrier in a shaft furnace to maintain the reaction temperature and improve the effectiveness of the reduction of iron ores.
Outlook: Creating green ammonia and adjusting the iron reduction procedure
A sustainable transition is paved by ammonia-based direct reduction, which links two of the most CO2-intensive industries—the steel and ammonia industries. The logistical and energy drawbacks of hydrogen are also eliminated by using ammonia, and already developed furnace technologies, such as shaft and electric arc furnaces, can be used with only minor modifications.
To speed up the ammonia-based reduction process for a variety of industrial applications, the Max Planck team will test various process parameters in the following step, such as temperature or gas mixture.
More information: Yan Ma et al, Reducing Iron Oxide with Ammonia: A Sustainable Path to Green Steel, Advanced Science (2023). DOI: 10.1002/advs.202300111