A single inhaled dose of COVID-19 and its derivatives treated or even prevented illness.
- Current anti-SARS-CoV-2 therapies work by binding to one of three binding sites on the spike protein.
- Because the new antiviral binds to all three locations on the spike protein, it is more effective than conventional treatments.
- Antivirals are also low-cost, simple to manufacture, do not necessitate elaborate supply chains with severe cold, and might potentially be self-administered.
A novel protein-based antiviral nasal spray created by Northwestern University, the University of Washington, and Washington University in St. Louis is moving toward Phase I human clinical trials to treat COVID-19.
The new protein therapies, which were developed computationally and refined in the lab, prevented infection by interfering with the virus’s capacity to enter cells. The top protein killed the virus with similar or better potency than antibody therapies having U.S. Food and Drug Administration Emergency Use Authorization status (FDA). Notably, the top protein neutralized all tested SARS-CoV-2 variants, which many clinical antibodies have not been able to do.
When researchers gave the medication to mice in the form of a nasal spray, they discovered that the best antiviral proteins lowered signs of infection — or even avoided infection entirely.

The findings were published in the journal Science Translational Medicine on April 12, 2022.
This research was led by Northwestern’s Michael Jewett, the University of Washington School of Medicine’s David Baker and David Veesler, and WashU’s Michael S. Diamond.
To begin, the researchers employed supercomputers to create proteins that could adhere to susceptible areas on the surface of the novel coronavirus, thereby targeting the spike protein. This research was first published in the journal Science in 2020.
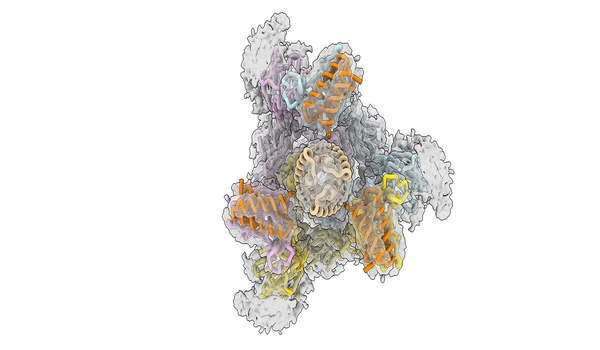
The team reengineered the proteins, known as minibinders, in the latest study to make them even more effective. Instead of targeting only one site of the virus’s infectious machinery, the minibinders bind to three sites at the same time, making the medicine less likely to detach.
“The spike protein of SARS-CoV-2 has three binding domains, and typical antibody treatments may only inhibit one,” Jewett explained. “Our minibinders act as a tripod on top of the spike protein, blocking all three.” The relationship between the spike protein and our antiviral is one of the most tightly bound in biology. When we combined the spike protein and our antiviral treatment in a test tube for a week, they remained attached and never separated.”
Jewett is the director of Northwestern’s Center for Synthetic Biology and a professor of chemical and biological engineering at Northwestern’s McCormick School of Engineering. The paper’s co-first author is Andrew C. Hunt, a graduate research fellow in Jewett’s laboratory.
Some medicines have grown less efficient in combating the ever-evolving SARS-CoV-2 virus as it has mutated to develop new strains. Just last month, the FDA halted many monoclonal antibody treatments, citing their failure to combat the BA.2 omicron subvariant.
Unlike the antibody therapies, which failed to neutralize omicron, the novel minibinders retained effectiveness against the omicron strain under consideration. The new antiviral blocks the virus from binding to the human angiotensin-converting enzyme 2 (ACE2) receptor, which is the virus’s entry point into the body, by disrupting its spike protein. Because the novel coronavirus and its mutant variations cannot infect the body unless they attach to the ACE2 receptor, the antiviral should be effective against future forms as well.
“The spike protein and the ACE2 receptor engage in a handshake to enter the body,” Jewett explained. “Our antiviral prevents this handshake and, as an added bonus, it is resistant to viral escape.”
“SARS-CoV-2’s spike protein has three binding domains, and common antibody therapies may only block one. Our mini binders sit on top of the spike protein like a tripod and block all three. The interaction between the spike protein and our antiviral is among the tightest interactions known in biology. When we put the spike protein and our antiviral therapeutic in a test tube together for a week, they stayed connected and never fell apart.”
Michael Jewett
Current antibody treatments, in addition to losing effectiveness, have significant drawbacks: They are difficult to create, costly, and must be administered by a healthcare practitioner. They also necessitate complex supply systems and intense refrigeration, both of which are frequently unavailable in low-resource areas.
All of these issues are addressed by the new antiviral. Unlike monoclonal antibodies, which are created by cloning and cultivating living human cells, the new antiviral medication is created on a massive scale in bacteria such as E. coli, making it more cost-effective to generate. Not only is the new therapy stable in high temperatures, which could help to expedite manufacture and lower the cost of goods for clinical research, but it also has the potential to be self-administered as a one-time nasal spray, eliminating the need for medical specialists.
The researchers envision it being sold at pharmacies and used as a preventative approach to cure infections.
Reference: “Multivalent designed proteins neutralize SARS-CoV-2 variants of concern and confer protection against infection in mice” by Andrew C. Hunt, James Brett Case, Young-Jun Park, Longxing Cao, Kejia Wu, Alexandra C. Walls, Zhuoming Liu, John E. Bowen, Hsien-Wei Yeh, Shally Saini, Louisa Helms, Yan Ting Zhao, Tien-Ying Hsiang, Tyler N. Starr, Inna Goreshnik, Lisa Kozodoy, Lauren Carter, Rashmi Ravichandran, Lydia B. Green, Wadim L. Matochko, Christy A. Thomson, Bastian Vögeli, Antje Krüger, Laura A. VanBlargan, Rita E. Chen, Baoling Ying, Adam L. Bailey, Natasha M. Kafai, Scott E. Boyken, Ajasja Ljubetic, Natasha Edman, George Ueda, Cameron M. Chow, Max Johnson, Amin Addetia, Mary Jane Navarro, Nuttada Panpradist, Michael Gale, Benjamin S. Freedman, Jesse D. Bloom, Hannele Ruohola-Baker, Sean P. J. Whelan, Lance Stewart, Michael S. Diamond, David Veesler, Michael C. Jewett and David Baker, 12 April 2022, Science Translational Medicine.





