Pancreatic ductal adenocarcinoma (PDAC) cells maintain an elevated degree of autophagy or corruption, permitting them to flourish in seriously restricting microenvironments. However, the mechanism by which autophagy aids in the growth and survival of pancreatic cancer cells remains a mystery.
Subhadip Mukhopadhyay and a team of researchers in radiation oncology and genome stability at NYU and Harvard demonstrated in a new Science Advances article how inhibiting autophagy altered mitochondrial function in PDAC patients. To restrict the labile iron pool’s availability, they experimentally attenuated the succinate dehydrogenase complex iron sulfur subunit in mitochondria.
The disease cell lines utilized autophagy to support iron homeostasis as opposed to other growth types that depended on macropinocytosis. The biologists observed that pancreatic cancer cells were able to resist autophagy ablation by receiving bioavailable iron from cancer-associated fibroblasts. In a mouse model, Mukhopadhyay and colleagues used autophagy inhibition therapy to prevent this crosstalk by giving the mice a diet low in iron. The findings highlighted a crucial connection that drives the progression of pancreatic ductal adenocarcinoma between autophagy, iron metabolism, and mitochondrial function.
Iron homeostasis in pancreatic ductal adenocarcinoma (PDAC)
Pancreatic ductal adenocarcinoma addresses over 95% of pancreatic diseases that are profoundly impervious to treatment with a low 5-year endurance rate, where the quantity of malignant growth-related deaths accounted for the second most elevated rate in the US. A harsh, hypoperfused, and hypoxic microenvironment with altered nutrient availability defines these tumors. The disease cells can endure antagonistic conditions by reconstructing their metabolic requirements to depend on supplement rummaging systems, for example, macroautophagy and macropinocytosis, to get by and develop. The selective form of autophagy known as ferritinophagy and its role in maintaining iron homeostasis have been the focus of previous research.
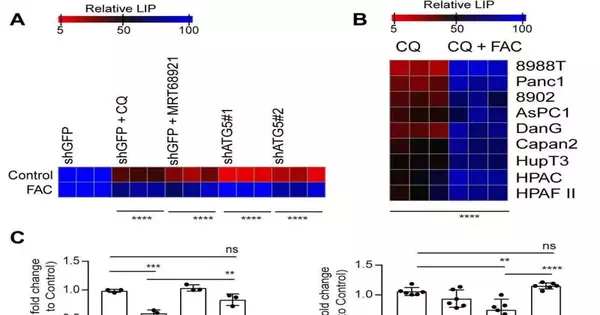
Autophagy restraint repeals SDHB levels in PDAC. In PDAC cells, pharmacological (A) or genetic (the meanB) inhibition of autophagy was combined with FAC for 24 hours, and immunoblots for the indicated proteins were performed. SDHB is brousingtilizing a triangle. Similar to (A), total ion counts of succinate in PDAC cells using liquid chroma-ography–mass spectrometry (n = 3 technical replicates) following autophagy inhibition were carried out using other PDAC cell lines in (C). The unpaired Student’s two-tailed t test was used to quantify P values, and the data are means standard deviation. P 0.001 and P 0.05 were deemed significant. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf9284
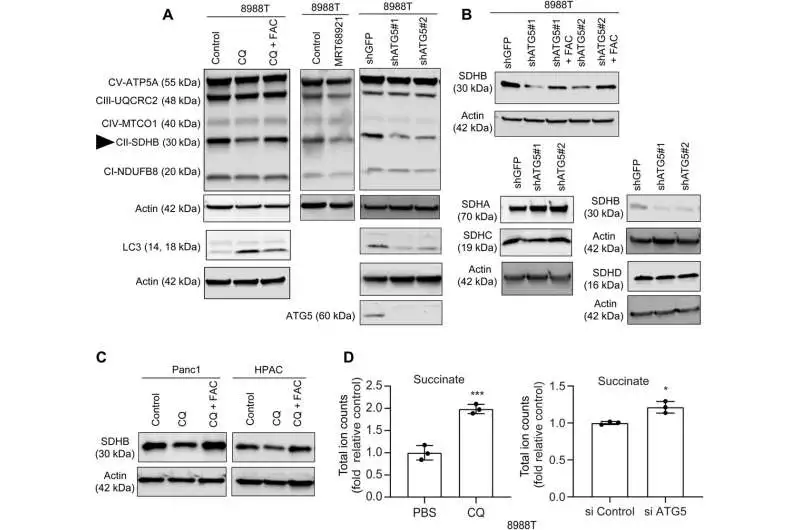
In this work, Mukhopadhyay and the group showed how the restraint of autophagy decreased the gathering of iron-sulfur bunches to settle different proteins, incorporating those engaged with the electron move chain, for example, succinate dehydrogenase complex iron sulfur subunit B (SDHB). During autophagy, this molecule is reduced in pancreatic cancer cells, resulting in a reorganization of the cristae microarchitecture and a decrease in mitochondrial respiration. The supplementation of iron or the outflow of ectopic SDHB restored development and worked on the mitochondrial deserts.
Inhibiting autophagy in pancreatic ductile cancer cells by reducing the amount of bioavailable iron Mukhopadhyay and colleagues demonstrated previously that inhibiting autophagy reduced the rate at which mitochondria consume oxygen. However, they were unable to identify the cause of this impairment. Earlier examination had shown amino corrosive pools to stay unaltered upon autophagy hindrance, with the exception of a diminishing in cysteine levels.
Nonetheless, enhancing cysteine in pancreatic disease cells didn’t change the flawed pace of oxygen utilization, demonstrating a pathway that straightforwardly impacted the mitochondria. While iron-sulfur group proteins drove mitochondrial capability, a drop in the labile iron pool after autophagy hindrance diminished mitochondrial breath, which the group reestablished by means of ferric ammonium citrate supplementation. The decreased levels of the succinate dehydrogenase complex iron sulfur subunit B (SDHB) protein revealed a functional loss of the mitochondrial protein as a result of the inhibition of autophagy.
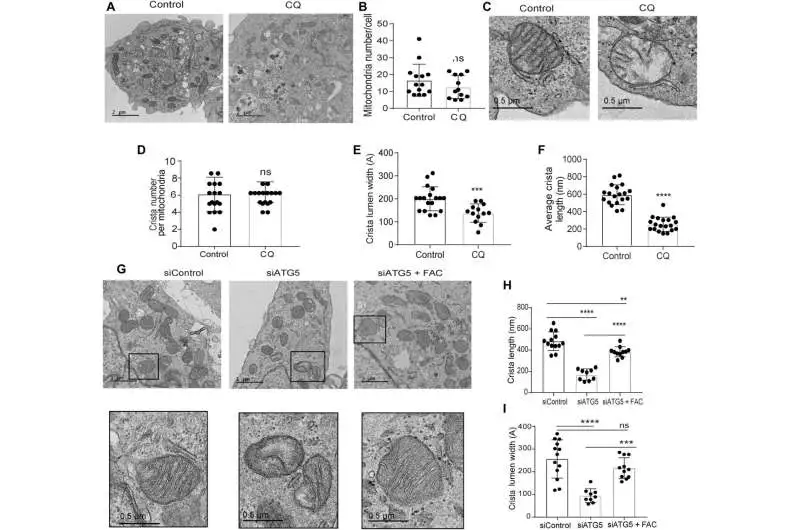
Loss of autophagy impedes mitochondrial microarchitecture in PDAC. (A) Delegate TEM pictures of PDAC cells treated with PBS (control) or CQ, followed by deciding their (B) mitochondrial number per cell (control, n = 14; CQ, 12 cells in total). C) An amplified segment of a PDAC cell showing mitochondrial ultrastructure after CQ treatment, followed by measurement of (D) crista number per mitochondria (n = 17 interesting crista for both Control and CQ), (E) crista lumen width (Control, n = 18), (F) crista length (n = 19 unique crista for both Control and CQ), and (CQ, n = 13 unique crista). G) Crista length (H) and lumen width (I) were measured in representative TEM images of ATG5-knockdown PDAC cells treated with or without FAC (siControl, 13; siATG5, n = 9; siATG5 and FAC, 11 distinct cristas). To bring out the typical morphology of dysfunctional mitochondria, black boxes were digitally zoomed in. The mitochondria were quantified using a random TEM image. The one-way ANOVA and Tukey’s post hoc test were used to quantify P values, and the data are means SD. ** Significant values were P 0.01, ***P 0.001, and ****P 0.0001. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf9284
Transmission electron microscopy was used to reveal the mitochondrial architecture of autophagy-deficient pancreatic cancer cells (10.1126/sciadv.adf9284). Defects in the mitochondrial architecture are related to autophagy inhibition empowered by directing chloroquine. After treatment, they observed a significant reduction in the lumen of the mitochondrial cristae and large empty spaces, which indicated functional defects.
The group demonstrated the way that the ectopic articulation of the SDHB protein could reestablish the cristae microarchitecture. After autophagy inhibition, proliferation and oxygen consumption rates were actually saved by SDHB overexpression. After pancreatic cancer cells lost autophagy, they investigated the mechanism by which these components played a significant role in the biogenesis of iron and sulfur proteins.
The patient’s response to hydroxychloroquine monotherapy was not clinically sufficient to attenuate the disease, despite the fact that autophagy can regulate the availability of iron in pancreatic cancer cells. This study is investigating the crosstalk between cancer-associated cell co-cultures and drug moieties. To inspect the effect of the growth microenvironment on this opposition, the specialists concentrated on the impacts of remunerating the labile iron pool by co-refined pancreatic disease cells with disease-related fibroblasts to reproduce a microphysiological cancer climate.
In order to comprehend the cellular regulation of the labile iron pool, the researchers looked at the expression of important iron transporters and the crosstalk between cells. When fibroblasts were cultured alongside autophagy-inhibited pancreatic cancer cells, they observed an increase in the exporter ferroprotein. They were able to restore the cancer cell line’s antiproliferative effects in the co-culture system after administering tocilizumab.
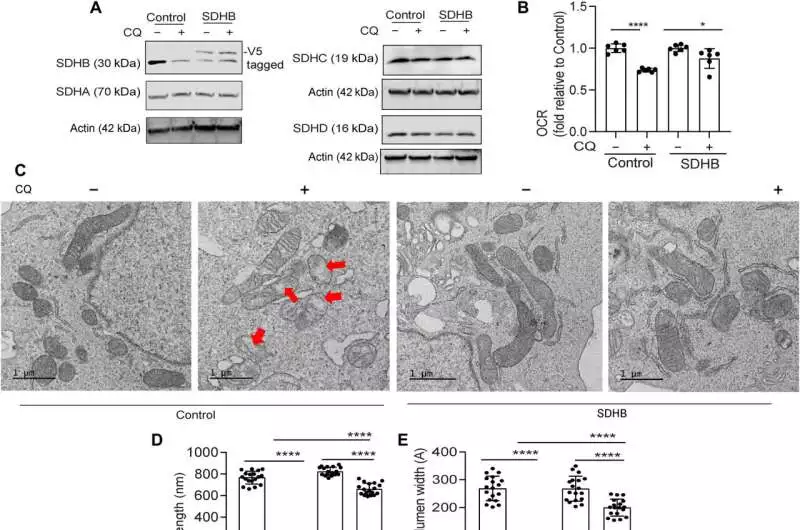
When PDAC inhibits autophagy, mitochondrial dysfunction is remedied by ectopic SDHB. After CQ treatment of PDAC cells expressing SDHB and the control vector, immunoblotting for the indicated proteins (A) and OCR (B) analysis (combined data from n = 2 experiments) were performed. C) Representative TEM images of cells in an environment similar to (A) are displayed, followed by the length of the mitochondrial crista (D; cristae (n = 18 for each group) and lumen width (E; n = 18 distinct cristae for each group) choice. In (C), typical mitochondrial dysfunction is depicted by red arrows. For immunoblotting of the indicated proteins, SDHB overexpression and control PDAC cells suppressed ATG5 (F). Under conditions like (F), cell expansion (G; combined results from five experiments) and OCR (H; combined results from two experiments) (n = one-way ANOVA with Tukey’s post hoc test was used to quantify P values, and the data are means standard deviation). Significant values were P 0.05, **P 0.01, ***P 0.001, and ****P 0.0001.Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf9284
Treating pancreatic ductal adenocarcinoma (PDAC)
Mukhopadhyay and partners investigated the techniques to treat the disease and if autophagy hindrance would work with iron limitation to restrain pancreatic cancer development. In order to hinder the communication between cancer-associated fibroblasts and adenocarcinoma cells in the pancreatic duct, they reduced the bioavailability of iron. They achieved this by deliberately lessening iron levels in mice by presenting them to a low-iron eating regimen for a long time prior to relocating malignant growth cells with a doxycycline-inducible predominant vector and a control vector in mice. While the iron level in the eating routine didn’t influence the degree of hemoglobin, autophagy hindrance essentially diminished cancer development. The low-iron eating routine further decreased cancer development, suggesting that iron hardship sharpened growth cells to go through autophagy hindrance.
Both an iron-deficient diet and autophagy reduced the expression of succinate dehydrogenase complex iron sulfur subunit B and the concentration of bioavailable ferrous iron in tumors. At the point when the researchers regulated chloroquine alone, it neglected to diminish cancer development in mice, albeit consolidated chloroquine and an iron-limited diet altogether decreased cancer development. The consolidated information addressed a low-iron eating regimen that cooperated with autophagy hindrance to fundamentally upset iron homeostasis in pancreatic ductal adenocarcinomas.
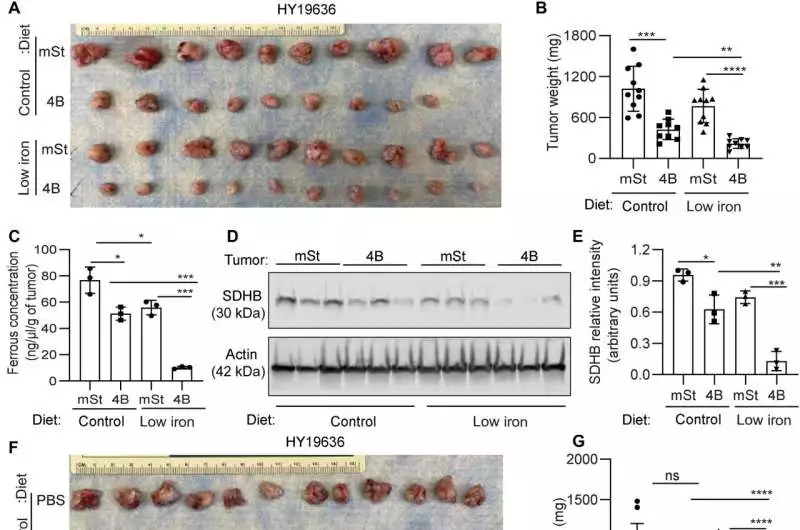
In PDAC, the combination of an iron-deficient diet and inhibiting autophagy has therapeutic benefits. A) Murine PDAC cells (HY19636) bearing flawless autophagy (mSt) or repressed autophagy (4B) were orthotopically embedded in syngeneic mice taken care of with doxycycline (625 mg/kg) and a diet containing control (220 ppm) or low iron (4 to 6 ppm). Mice were euthanized following 24 days of implantation, and growth pictures (A; from top to bottom) and tumor weights (B) are shown, respectively. C) Three tumors from each group were selected at random, and their ferrous concentrations were examined. (A) (D) Immunoblot analysis of these tumors revealed SDHB expression levels, and their relative quantification was carried out (E). B6 mice bearing syngeneic PDAC cells were treated with CQ or PBS, taken care of with a control or low-iron eating regimen, and were euthanized 24 days after implantation. The images of tumors (F; from top to bottom, n = 12, 10, 12, and 12), as well as tumor weights (G), H) The ferrous concentration in the tumors of the three groups indicated was determined. I) SDHB articulation level was surveyed by immunoblotting on these growths, and their general measurement was performed (J) (n = 3). Information implies SD, and P values were measured utilizing one-way ANOVA with Tukey’s post hoc test. * Significant values were P 0.05, **P 0.01, ***P 0.001, and ****P 0.0001. Credit: Science Advances (2023). DOI: 10.1126/sciadv.adf9284.
Subhadip Mukhopadhyay and colleagues discovered this way how autophagy was used by pancreatic duct adenocarcinoma tumors to grow and change over time as the disease progressed. The synthesis of iron-sulfur clusters and the labile iron pool were both reduced when autophagy was inhibited. To restore mitochondrial function and cell growth, they added iron to the cultures and encouraged the ectopic expression of both iron-sulfur clusters and the succinate dehydrogenase complex iron sulfur subunit B (SDHB) protein. In pancreatic cancer cells, autophagy is necessary to support mitochondrial function, which is necessary for growth and proliferation, and to maintain iron homeostasis.
The researchers examined the disruption of iron metabolism in the pancreatic duct adenocarcinoma cells and its effect on the complex tumor microenvironment, as well as the numerous metabolic cross-talks that took place between the tumor and the stroma. New design strategies to target the iron metabolism process in pancreatic duct adenocarcinoma cells will benefit from these mechanistic findings.
More information: Subhadip Mukhopadhyay et al, Autophagy supports mitochondrial metabolism through the regulation of iron homeostasis in pancreatic cancer, Science Advances (2023). DOI: 10.1126/sciadv.adf9284
Cristovão M. Sousa et al, Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion, Nature (2016). DOI: 10.1038/nature19084