Under ordinary circumstances, radioactive materials, for example, uranium, work in an anticipated way.
Take those equivalent materials and subject them to extraordinary conditions, such as high temperatures in a short timescale and a rapid cooling process, and their decay pathways change dramatically.
Lawrence Livermore National Laboratory (LLNL) researchers constructed an exceptional cycle to blend radioactive mixtures (uranium-based) that are incredibly air- and water-delicate and require explicit methods. The team was then able to simulate how these mixtures would behave under extreme conditions by using a specially designed laser chamber designed for dealing with radioactive material.
“The knowledge could be used in materials manufacture, stockpile management, or even garbage consolidation, processing, or storage. We could envisage storing a metal-containing molecule in a stable state and then reacting it with lasers to create a new product.”
LLNL radiochemist Maryline Kerlin (Ferrier), first author of the paper.
Because response rates are so fast and thus distant from balance processes, this work investigated new response pathways for warm deterioration.The exploration shows up on the front page of inorganic science.

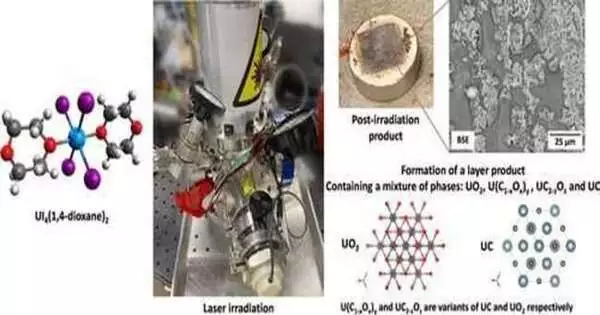
An orange powder, UI4(1,4-dioxane)2, is thermally disintegrating under laser illumination to frame a slender layer of material containing a combination of degradation items (i.e., UO2, U(C1-xOx)y, UC2-zOz, and UC). The outrageous idea of the laser light cycle is seen in the creation of a fume tuft during the serious temperature cycling.
Up to this point, researchers didn’t have a decent comprehension of the science related to the warming deterioration of receptive coordination, which intensified under outrageous circumstances.
The cycle could be compared to placing water on a griddle. Assuming you gradually warm up the water, it behaves nicely and bubbles gradually.Notwithstanding, on the off chance that you drop water on a hot skillet (similar to the laser), the response is totally different, and the water quickly disintegrates.
“The information might possibly be applied to materials production, reserve stewardship, or even waste combination, handling, or stockpiling,” said LLNL radiochemist Maryline Kerlin (Ferrier), the first creator of the paper. “We could envision putting away a metal-containing compound under a steady setup and then responding to it under lasers to get another item.”
The investigation of metal-containing intensifiers that contain natural ligands is typically conducted under gentle circumstances, since these mixtures are known to be very delicate to air, water, and temperature. As a rule, working with these mixtures requires an exceptionally scholarly way to deal with their construction, electronic design, synthetic reactivity, properties, and so on. Thus, it could appear to be whimsical to warm these mixtures forcefully.
In any case, the group needed to comprehend assuming that it was feasible to sidestep standard sorts of responses and degradation courses.
“We needed to see if, by utilizing outrageous conditions, it was feasible to make new pathways to change the forerunner’s build into an alternate accumulate of interest, like uranium metal or uranium carbides,” Kerlin said. “Past work has been directed in the past utilizing laser-driven science, but this is the first time that the forerunner first utilized a uranium-based compound containing natural ligands (i.e., air- and water-soluble).”
These discoveries are significant on the grounds that they showed that the forerunner didn’t act true to form. Warm decay utilizing a laser does, as a matter of fact, produce different response rates and pathways. The group didn’t acquire uranium metal true to form; however, the thermally deteriorated encompassing ligands permitted it to frame carbide and oxy-carbide, which deliberately work in the end result. Understanding uranium science is significant for store stewardship and waste handling.
“New strategies and courses to deliver helpful earthenware production and metals are applicable for metals other than uranium, and that information can measure up to all the more commonly utilized metal mixtures,” Kerlin said. “The ultimate objective (i.e., making uranium metal or earthenware production) drove the beginning of this exploration, yet the cycle applies to materials that we use in our day-to-day routines.”
More information: Maryline G. Ferrier et al, Unconventional Pathways to Carbide Phase Synthesis via Thermal Decomposition of UI4(1,4-dioxane)2, Inorganic Chemistry (2022). DOI: 10.1021/acs.inorgchem.2c02590
Journal information: Inorganic Chemistry